Characterization of Saccharides in Size-Fractionated Ambient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Universidad De Concepción Facultad De Ciencias Naturales Y
Universidad de Concepción Facultad de Ciencias Naturales y Oceanográficas Programa de Magister en Ciencias mención Botánica DIVERSIDAD DE MACROHONGOS EN ÁREAS DESÉRTICAS DEL NORTE GRANDE DE CHILE Tesis presentada a la Facultad de Ciencias Naturales y Oceanográficas de la Universidad de Concepción para optar al grado de Magister en Ciencias mención Botánica POR: SANDRA CAROLINA TRONCOSO ALARCÓN Profesor Guía: Dr. Götz Palfner Profesora Co-Guía: Dra. Angélica Casanova Mayo 2020 Concepción, Chile AGRADECIMIENTOS Son muchas las personas que han contribuido durante el proceso y conclusión de este trabajo. En primer lugar quiero agradecer al Dr. Götz Palfner, guía de esta tesis y mi profesor desde el año 2014 y a la Dra. Angélica Casanova, quienes con su experiencia se han esforzado en enseñarme y ayudarme a llegar a esta instancia. A mis compañeros de laboratorio, Josefa Binimelis, Catalina Marín y Cristobal Araneda, por la ayuda mutua y compañía que nos pudimos brindar en el laboratorio o durante los viajes que se realizaron para contribuir a esta tesis. A CONAF Atacama y al Proyecto RT2716 “Ecofisiología de Líquenes Antárticos y del desierto de Atacama”, cuyas gestiones o financiamientos permitieron conocer un poco más de la diversidad de hongos y de los bellos paisajes de nuestro desierto chileno. Agradezco al Dr. Pablo Guerrero y a todas las personas que enviaron muestras fúngicas desertícolas al Laboratorio de Micología para identificarlas y que fueron incluidas en esta investigación. También agradezco a mi familia, mis padres, hermanos, abuelos y tíos, que siempre me han apoyado y animado a llevar a cabo mis metas de manera incondicional. -

A New Section and Two New Species of Podaxis (Gasteromycetes) from South Africa
S.Afr.l. Bot., 1989,55(2): 159-164 159 A new section and two new species of Podaxis (Gasteromycetes) from South Africa J.J.R. de Villiers, A. Eicker* and G.C.A. van der Westhuizen Department of Botany, University of Pretoria, Pretoria, 0001 Republic of South Africa Accepted 7 September /988 Podaxis africana De Villiers, Eicker & van der Westhuizen and P. rugospora De Villiers, Eicker & van der Westhuizen, two new species from Transvaal are described and illustrated. A new section, Umbricorticalis De Villiers, Eicker & van der Westhuizen is proposed in the genus Podaxis to accommodate P. africana. Morphologically P. africana resembles P. microporus McKnight but it is distinguished by its large globose, subglobose to broadly ovoid spores, the absence of a 'pin prick' pore structure, the black gleba, and the deep orange to strong brown inner cortex of the stipe. P. rugospora is allied to P. pistillaris (L. ex Pers.) Fr. emend. Morse from which it differs by reason of the hyaline, narrow, flattened, occasionally septate capillitium threads and the grayish-olive, light olive or light to moderate yellowish-brown gleba. The most remarkable character of these new species is the rugose spores. Podaxis africana De Villiers, Eicker & van der Westhuizen en P. rugospora De Villiers, Eicker & van der Westhuizen, twee nuwe spesies van Transvaal word beskryf en ge·illustreer. 'n Nuwe seksie, Umbricorticalis De Villiers, Eicker & van der Westhuizen in die genus Podaxis word voorgestel om P. africana te akkommodeer. Morfologies toon P. africana 'n ooreenkoms met P. microporus McKnight maar word deur die groot bolvormige, subbolvormige tot bykans eiervormige spore, die afwesigheid van 'n 'naaldprik'-porie struktuur, die swart gleba en die diep oranje tot sterk bruin binneste korteks van die stipe. -

Qrno. 1 2 3 4 5 6 7 1 CP 2903 77 100 0 Cfcl3
QRNo. General description of Type of Tariff line code(s) affected, based on Detailed Product Description WTO Justification (e.g. National legal basis and entry into Administration, modification of previously the restriction restriction HS(2012) Article XX(g) of the GATT, etc.) force (i.e. Law, regulation or notified measures, and other comments (Symbol in and Grounds for Restriction, administrative decision) Annex 2 of e.g., Other International the Decision) Commitments (e.g. Montreal Protocol, CITES, etc) 12 3 4 5 6 7 1 Prohibition to CP 2903 77 100 0 CFCl3 (CFC-11) Trichlorofluoromethane Article XX(h) GATT Board of Eurasian Economic Import/export of these ozone destroying import/export ozone CP-X Commission substances from/to the customs territory of the destroying substances 2903 77 200 0 CF2Cl2 (CFC-12) Dichlorodifluoromethane Article 46 of the EAEU Treaty DECISION on August 16, 2012 N Eurasian Economic Union is permitted only in (excluding goods in dated 29 may 2014 and paragraphs 134 the following cases: transit) (all EAEU 2903 77 300 0 C2F3Cl3 (CFC-113) 1,1,2- 4 and 37 of the Protocol on non- On legal acts in the field of non- _to be used solely as a raw material for the countries) Trichlorotrifluoroethane tariff regulation measures against tariff regulation (as last amended at 2 production of other chemicals; third countries Annex No. 7 to the June 2016) EAEU of 29 May 2014 Annex 1 to the Decision N 134 dated 16 August 2012 Unit list of goods subject to prohibitions or restrictions on import or export by countries- members of the -

A Note on Battarrea Phalloides in Turkey
MANTAR DERGİSİ/The Journal of Fungus Nisan(2021)12(1)1-9 Geliş(Recevied) :26.09.2020 Research Article Kabul(Accepted) :12.11.2020 Doi: 10.30708.mantar.800585 A Note on Battarrea phalloides in Turkey 1*, 2 1 Ilgaz AKATA Deniz ALTUNTAŞ , Ergin ŞAHİN , Hakan ALLI3, ŞANLI KABAKTEPE4 *Corresponding author: [email protected] 1 Ankara University, Faculty of Science, Department of Biology, Ankara, Turkey Orcid ID: 0000-0002-1731-1302/ [email protected] Orcid ID: 0000-0003-1711-738X/ [email protected] 2Ankara University, Graduate School of Natural and Applied Sciences, Ankara, Turkey Orcid ID: 0000-0003-0142-6188/ [email protected] 3Muğla Sıtkı Koçman University, Faculty of Science, Department of Biology, Muğla, Turkey Orcid ID: 0000-0001-8781-7089/ [email protected] 4Malatya Turgut Ozal University, Battalgazi Vocat Sch., Battalgazi, Malatya, Turkey Orcid ID: 0000-0001-8286-9225/[email protected] Abstract: The current study was conducted based on a Battarrea sample obtained from Muğla province (Turkey). The sample was identified based on both conventional methods and ITS rDNA region-based molecular phylogeny. By taking into account the high sequence similarity between the sample (ANK Akata & Altuntaş 690) and Battarrea phalloides the relevant specimen was considered to be B. Phalloides and the morphological data also strengthen this finding. In this study, photos of macro and microscopic structures, a short description, scanning electron microscope (SEM) images of spores and elaters, and the ITS rDNA region-based molecular phylogeny of the samples were given. Also, the distribution of B. phalloides specimens identified thus far from Turkey was revealed for the first time in this study. -

Research Article
Marwa H. E. Elnaiem et al / Int. J. Res. Ayurveda Pharm. 8 (Suppl 3), 2017 Research Article www.ijrap.net MORPHOLOGICAL AND MOLECULAR CHARACTERIZATION OF WILD MUSHROOMS IN KHARTOUM NORTH, SUDAN Marwa H. E. Elnaiem 1, Ahmed A. Mahdi 1,3, Ghazi H. Badawi 2 and Idress H. Attitalla 3* 1Department of Botany & Agric. Biotechnology, Faculty of Agriculture, University of Khartoum, Sudan 2Department of Agronomy, Faculty of Agriculture, University of Khartoum, Sudan 3Department of Microbiology, Faculty of Science, Omar Al-Mukhtar University, Beida, Libya Received on: 26/03/17 Accepted on: 20/05/17 *Corresponding author E-mail: [email protected] DOI: 10.7897/2277-4343.083197 ABSTRACT In a study of the diversity of wild mushrooms in Sudan, fifty-six samples were collected from various locations in Sharq Elneel and Shambat areas of Khartoum North. Based on ten morphological characteristics, the samples were assigned to fifteen groups, each representing a distinct species. Eleven groups were identified to species level, while the remaining four could not, and it is suggested that they are Agaricales sensu Lato. The most predominant species was Chlorophylum molybdites (15 samples). The identified species belonged to three orders: Agaricales, Phallales and Polyporales. Agaricales was represented by four families (Psathyrellaceae, Lepiotaceae, Podaxaceae and Amanitaceae), but Phallales and Polyporales were represented by only one family each (Phallaceae and Hymenochaetaceae, respectively), each of which included a single species. The genetic diversity of the samples was studied by the RAPD-PCR technique, using six random 10-nucleotide primers. Three of the primers (OPL3, OPL8 and OPQ1) worked on fifty-two of the fifty-six samples and gave a total of 140 bands. -

A Preliminary Checklist of Arizona Macrofungi
A PRELIMINARY CHECKLIST OF ARIZONA MACROFUNGI Scott T. Bates School of Life Sciences Arizona State University PO Box 874601 Tempe, AZ 85287-4601 ABSTRACT A checklist of 1290 species of nonlichenized ascomycetaceous, basidiomycetaceous, and zygomycetaceous macrofungi is presented for the state of Arizona. The checklist was compiled from records of Arizona fungi in scientific publications or herbarium databases. Additional records were obtained from a physical search of herbarium specimens in the University of Arizona’s Robert L. Gilbertson Mycological Herbarium and of the author’s personal herbarium. This publication represents the first comprehensive checklist of macrofungi for Arizona. In all probability, the checklist is far from complete as new species await discovery and some of the species listed are in need of taxonomic revision. The data presented here serve as a baseline for future studies related to fungal biodiversity in Arizona and can contribute to state or national inventories of biota. INTRODUCTION Arizona is a state noted for the diversity of its biotic communities (Brown 1994). Boreal forests found at high altitudes, the ‘Sky Islands’ prevalent in the southern parts of the state, and ponderosa pine (Pinus ponderosa P.& C. Lawson) forests that are widespread in Arizona, all provide rich habitats that sustain numerous species of macrofungi. Even xeric biomes, such as desertscrub and semidesert- grasslands, support a unique mycota, which include rare species such as Itajahya galericulata A. Møller (Long & Stouffer 1943b, Fig. 2c). Although checklists for some groups of fungi present in the state have been published previously (e.g., Gilbertson & Budington 1970, Gilbertson et al. 1974, Gilbertson & Bigelow 1998, Fogel & States 2002), this checklist represents the first comprehensive listing of all macrofungi in the kingdom Eumycota (Fungi) that are known from Arizona. -

Podaxis Pistillaris Reported from Madhya Pradesh, India Patel U
Indian Journal of Fundamental and Applied Life Sciences ISSN: 2231-6345 (Online) An Online International Journal Available at http://www.cibtech.org/jls.htm 2012 Vol. 2 (1) January- March, pp.233 -239 /Patel et al. Research Article PODAXIS PISTILLARIS REPORTED FROM MADHYA PRADESH, INDIA PATEL U. S.1 and *TIWARI A. K.2 Department of Botany, Government Shyam Sunder Agrawal College, Sihora, District Jabalpur, Madhya Pradesh, India *2 Jabalpur Institute of Nursing Science & Research, Jabalpur, Madhya Pradesh, India *Author for Correspondence ABSTRACT A basidiomycete collected from Sihora, District Jabalpur is described and illustrated. The fruiting bodies and spores showed variations however, match with the description of Podaxis pistillaris. This is first report of the fungus from Madhya Pradesh, India. Key Words: Comatus, Agaricaceae, Lycoperdaceae, Food, Medicinal INTRODUCTION Sihora road railway station is situated in Jabalpur district of Madhya Pradesh about 17 kilometers south west from Karaundi the center point of India. Its geographical co-ordinates are 230 29’ North and 800 70’ East. Some cylindrical fruiting bodies were incidentally seen on disturbed soil of filling of a pond beside the road going towards Khitola bus stand from railway station. The fungus was initially identified as Coprinus comatus (O. F. Mull.) Pers. Later it was identified as Podaxis pistillaris (L. Pers.) Morse.This fungus is commonly known as False Shaggy Mane due to its resemblance with C. comatus. This species has previously been described and synonymised by few workers (Massee, 1890; Morse, 1933, 1941; Keirle et al., 2004; Drechsler- Santosh , 2008). Podaxis pistillaris earlier has been reported from various parts of India (Bilgrami et al., 1979,81,1991; Jamaluddin et al., 2004). -
This Poster I It's Designed Large the Placeho Formatted Fo Placeholder
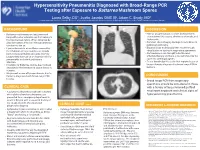
Hypersensitivity Pneumonitis Diagnosed with Broad-Range PCR Testing after Exposure to Battarrea Mushroom Spores • Laura Selby, DO1, Justin Jacobs OMS III2, Adam C. Brady, MD1 Printing: 1. Samaritan Health Services, Corvallis, Oregon 2. Western University of Health Sciences College of Osteopathic Medicine of the Pacific Northwest , Lebanon, Oregon This poster is 48” wide by 36” high. It’s designed to be printed on a BACKGROUND DISCUSSION large • Battarrea mushrooms are long-stemmed • Human lycoperdonosis is a rare disease that is fungi with a characteristic cap that belong to characterized by nausea, shortness of breath, and the Agaricaceae family. When disturbed by tachycardia. physical contact, they can release numerous • Common chest imaging findings include bilateral Customizing the Content: conidia into the air. pulmonary infiltrates. • Lycoperdonosis is a rare illness caused by • Diagnosis has traditionally been made through The placeholders in this the inhalation of large numbers of conidia visualization of spores in respiratory specimens. from certain puffball mushrooms (namely • Corticosteroids are thought to be the most formatted for you. Lycoperdon) that can cause hypersensitivity effective therapy and there is no definitive role for placeholders to add text, or click pneumonitis and mimic pulmonary systemic antifungal agents. infection. • To our knowledge this is the first reported case of an icon to add a table, chart, • Inhalation of Battarrea conidia has not been lycoperdonosis diagnosed by broad range PCR in described in the literature to cause illness in humans. SmartArt graphic, picture or humans. multimedia file. • We present a case of lycoperdonosis due to Battarea, diagnosed with broad-range PCR CONCLUSION T testing. -

CV Battarrea Phalloides (Dicks
Science & Technologies NEW DATA OF SOME RARE LARGER FUNGI OF AGARICACEAE (AGARICALES) IN BULGARIA Maria Lacheva Agricultural University-Plovdiv 12, Mendeleev Str., 4000 Plovdiv, Bulgaria E-mail: [email protected] ABSTRACT New data on seventeen rare macromycetous species from Agaricaceae are reported. Seven of them – Agaricus altipes, A. bohusii, A. macrocarpus, Battarrea phalloides, Chlorophyllum agaricoides, Tulostoma fimbriatum and T. volvulatum are of high conservation value included in the Red List of fungi in Bulgaria. All taxa are presented with brief chorological data and notes on their distribution in the country. Presented are macroscopic pictures of species of conservation value. Kew words: Agaricaceae, chorological data, conservation value, rare species, Red List. INTRODUCTION Mycological analysis of the literature shows that the diversity of Agaricaceae in the country is relatively low studied. The paper presents new chorological data for eighteen rare fungi belonging to Agaricaceae in Bulgaria. Seven species have conservation value included in the Red List of Fungi in Bulgaria (Gyosheva et al., 2006). MATERIALS AND METHODS The macromycetes were registered during mycological field trips in differently floristic regions of the country. Distribution of the taxa is given according to the floristic regions adopted in the Flora of the PR Bulgaria (Jordanov, 1966) [1] Black Sea Coast, [2] Northeast Bulgaria, [3] Danubian Plain, [4] Forebalkan, [5] Stara Planina Mts (western, central, eastern), [6] Sofia region, [7] Znepole region, [8] Vitosha region, [9] West Frontier Mts, [10] Valley of Struma River, [11] Mt Belasitsa, [12] Mt Slavyanka, [13] Valley of Mesta River, [14] Pirin Mts, [15] Rila Mts, [16] Mt Sredna Gora (western, eastern), [17] Rhodopi Mts (western, central, eastern), [18] Thracian Lowland, [19] Tundzha Hilly Country, [20] Mt Strandzha. -

New Records on the Genus Tomophagus and Battarrea for Mycobiota of Egypt
Current Research in Environmental & Applied Mycology (Journal of Fungal Biology) 9(1): 77–84 (2019) ISSN 2229-2225 www.creamjournal.org Article Doi 10.5943/cream/9/1/8 New records on the genus Tomophagus and Battarrea for mycobiota of Egypt Abdel-Azeem AM1* and Nafady NA2 1Department of Botany, Faculty of Science, University of Suez Canal, Ismailia 41522, Egypt 2Department of Botany and Microbiology, Faculty of Science, University of Assiut, Assiut 71516, Egypt Abdel-Azeem AM, Nafady NA 2019 – New records on the genus Tomophagus and Battarrea for mycobiota of Egypt. Current Research in Environmental & Applied Mycology (Journal of Fungal Biology) 9(1), 77–84, Doi 10.5943/cream/9/1/8 Abstract During an extensive survey of macrobasidiomycota and the effects of climate changes on their distribution supported by Alexandria Research Center for Adaptation (ARCA) in Egypt and Mohamed bin Zayed Species Conservation Fund (MBZ), several specimens collected, examined and preserved. As a result, two species of Tomophagus colossus (Fr.) Murrill (Basidiomycota, Ganodermataceae) and Battarrea phalloides (Dicks.) Pers. (Basidiomycota, Agaricaceae) were identified and recorded as new records. Both taxa were identified phenotypically and were subjected to sequencing for confirmation. The internal transcribed spacer (ITS) 1–5.8 s – ITS2 rDNA sequences obtained were compared with those deposited in the GenBank Database and registered with accession number MH796120 and MH796121 in the NCBI Database respectively. We provide an updated full description and illustration of both species. Key words – Agaricaceae – ARCA – Basidiomycota – Ganodermataceae – Ismailia – MBZ – Nile delta Introduction Ganodermataceae Donk (Basidiomycota) was described in 1948 on the basis of double walled basidiospores, with an outer (exosporium) layer relatively thin and hyaline, and the inner (endosporium) usually pigmented, thick and often ornamented, rarely smooth (Cannon & Kirk 2007). -

Fungi from the Owyhee Region
FUNGI FROM THE OWYHEE REGION OF SOUTHERN IDAHO AND EASTERN OREGON bY Marcia C. Wicklow-Howard and Julie Kaltenecker Boise State University Boise, Idaho Prepared for: Eastside Ecosystem Management Project October 1994 THE OWYHEE REGION The Owyhee Region is south of the Snake River and covers Owyhee County, Idaho, Malheur County, Oregon, and a part of northern Nevada. It extends approximately from 115” to 118” West longitude and is bounded by parallels 41” to 44”. Owyhee County includes 7,662 square miles, Malheur County has 9,861 square miles, and the part of northern Nevada which is in the Owyhee River watershed is about 2,900 square miles. The elevations in the region range from about 660 m in the Snake River Plains and adjoining Owyhee Uplands to 2522 m at Hayden Peak in the Owyhee Mountains. Where the Snake River Plain area is mostly sediment-covered basalt, the area south of the Snake River known as the Owyhee Uplands, includes rolling hills, sharply dissected by basaltic plateaus. The Owyhee Mountains have a complex geology, with steep slopes of both basalt and granite. In the northern areas of the Owyhee Mountains, the steep hills, mountains, and escarpments consist of basalt. In other areas of the mountains the steep slopes are of granitic or rhyolitic origin. The mountains are surrounded by broad expanses of sagebrush covered plateaus. The soils of the Snake River Plains are generally non-calcareous and alkaline. Most are well-drained, with common soil textures of silt loam, loam and fine sand loam. In the Uplands and Mountains, the soils are often coarse textured on the surface, while the subsoils are loamy and non-calcareous. -

Reviewing the Taxonomy of Podaxis: Opportunities for Understanding Extreme Fungal Lifestyles
Fungal Biology 123 (2019) 183e187 Contents lists available at ScienceDirect Fungal Biology journal homepage: www.elsevier.com/locate/funbio Review Reviewing the taxonomy of Podaxis: Opportunities for understanding extreme fungal lifestyles * Benjamin H. Conlon a, , Duur K. Aanen b, Christine Beemelmanns c, Z. Wilhelm de Beer d, Henrik H. De Fine Licht e, Nina Gunde-Cimerman f, Morten Schiøtt a, Michael Poulsen a a Section for Ecology and Evolution, Department of Biology, University of Copenhagen, Copenhagen, Denmark b Laboratory of Genetics, Plant Sciences Group, Wageningen University, Netherlands c Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knoll-Institute,€ Chemical Biology, Jena, Germany d Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa e Department of Plant and Environmental Sciences, University of Copenhagen, Denmark f Department of Biology, Biotechnical Faculty, University of Ljubljana, Ljubljana, Slovenia article info abstract Article history: There are few environments more hostile and species-poor than deserts and the mounds of Nasuti- Received 27 August 2018 termitinae termites. However, despite the very different adaptations required to survive in such extreme Received in revised form and different environments, the fungal genus Podaxis is capable of surviving in both: where few other 13 December 2018 fungi are reported to grow. Despite their prominence in the landscape and their frequent documentation Accepted 8 January 2019 by early explorers, there has been relatively little research into the genus. Originally described by Available online 11 January 2019 Linnaeus in 1771, in the early 20th Century, the then ~25 species of Podaxis were almost entirely reduced Corresponding Editor: Ursula Peintner into one species: Podaxis pistillaris.