A Note on Battarrea Phalloides in Turkey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Universidad De Concepción Facultad De Ciencias Naturales Y
Universidad de Concepción Facultad de Ciencias Naturales y Oceanográficas Programa de Magister en Ciencias mención Botánica DIVERSIDAD DE MACROHONGOS EN ÁREAS DESÉRTICAS DEL NORTE GRANDE DE CHILE Tesis presentada a la Facultad de Ciencias Naturales y Oceanográficas de la Universidad de Concepción para optar al grado de Magister en Ciencias mención Botánica POR: SANDRA CAROLINA TRONCOSO ALARCÓN Profesor Guía: Dr. Götz Palfner Profesora Co-Guía: Dra. Angélica Casanova Mayo 2020 Concepción, Chile AGRADECIMIENTOS Son muchas las personas que han contribuido durante el proceso y conclusión de este trabajo. En primer lugar quiero agradecer al Dr. Götz Palfner, guía de esta tesis y mi profesor desde el año 2014 y a la Dra. Angélica Casanova, quienes con su experiencia se han esforzado en enseñarme y ayudarme a llegar a esta instancia. A mis compañeros de laboratorio, Josefa Binimelis, Catalina Marín y Cristobal Araneda, por la ayuda mutua y compañía que nos pudimos brindar en el laboratorio o durante los viajes que se realizaron para contribuir a esta tesis. A CONAF Atacama y al Proyecto RT2716 “Ecofisiología de Líquenes Antárticos y del desierto de Atacama”, cuyas gestiones o financiamientos permitieron conocer un poco más de la diversidad de hongos y de los bellos paisajes de nuestro desierto chileno. Agradezco al Dr. Pablo Guerrero y a todas las personas que enviaron muestras fúngicas desertícolas al Laboratorio de Micología para identificarlas y que fueron incluidas en esta investigación. También agradezco a mi familia, mis padres, hermanos, abuelos y tíos, que siempre me han apoyado y animado a llevar a cabo mis metas de manera incondicional. -

Qrno. 1 2 3 4 5 6 7 1 CP 2903 77 100 0 Cfcl3
QRNo. General description of Type of Tariff line code(s) affected, based on Detailed Product Description WTO Justification (e.g. National legal basis and entry into Administration, modification of previously the restriction restriction HS(2012) Article XX(g) of the GATT, etc.) force (i.e. Law, regulation or notified measures, and other comments (Symbol in and Grounds for Restriction, administrative decision) Annex 2 of e.g., Other International the Decision) Commitments (e.g. Montreal Protocol, CITES, etc) 12 3 4 5 6 7 1 Prohibition to CP 2903 77 100 0 CFCl3 (CFC-11) Trichlorofluoromethane Article XX(h) GATT Board of Eurasian Economic Import/export of these ozone destroying import/export ozone CP-X Commission substances from/to the customs territory of the destroying substances 2903 77 200 0 CF2Cl2 (CFC-12) Dichlorodifluoromethane Article 46 of the EAEU Treaty DECISION on August 16, 2012 N Eurasian Economic Union is permitted only in (excluding goods in dated 29 may 2014 and paragraphs 134 the following cases: transit) (all EAEU 2903 77 300 0 C2F3Cl3 (CFC-113) 1,1,2- 4 and 37 of the Protocol on non- On legal acts in the field of non- _to be used solely as a raw material for the countries) Trichlorotrifluoroethane tariff regulation measures against tariff regulation (as last amended at 2 production of other chemicals; third countries Annex No. 7 to the June 2016) EAEU of 29 May 2014 Annex 1 to the Decision N 134 dated 16 August 2012 Unit list of goods subject to prohibitions or restrictions on import or export by countries- members of the -

A Unique Signal Distorts the Perception of Species Richness
Microb Ecol DOI 10.1007/s00248-013-0266-4 SHORT COMMENTARY A Unique Signal Distorts the Perception of Species Richness and Composition in High-Throughput Sequencing Surveys of Microbial Communities: a Case Study of Fungi in Indoor Dust Rachel I. Adams & Anthony S. Amend & John W. Taylor & Thomas D. Bruns Received: 24 January 2013 /Accepted: 8 July 2013 # The Author(s) 2013. This article is published with open access at Springerlink.com Abstract Sequence-based surveys of microorganisms in sequenced to an even depth. Next, we used in silico manip- varied environments have found extremely diverse assem- ulations of the observed data to confirm that a unique signa- blages. A standard practice in current high-throughput se- ture can be identified with HTS approaches when the source quence (HTS) approaches in microbial ecology is to se- is abundant, whether or not the taxon identity is distinct. quence the composition of many environmental samples at Lastly, aerobiology of indoor fungi is discussed. once by pooling amplicon libraries at a common concentra- tion before processing on one run of a sequencing platform. Biomass of the target taxa, however, is not typically deter- mined prior to HTS, and here, we show that when abun- Introduction dances of the samples differ to a large degree, this standard practice can lead to a perceived bias in community richness Use of high-throughput sequencing (HTS) has transformed and composition. Fungal signal in settled dust of five uni- the field of microbial ecology. Traditionally, sampling depth, versity teaching laboratory classrooms, one of which was defined both in terms of the number of samples and intensity used for a mycology course, was surveyed. -

Download Download
LITERATURE UPDATE FOR TEXAS FLESHY BASIDIOMYCOTA WITH NEW VOUCHERED RECORDS FOR SOUTHEAST TEXAS David P. Lewis Clark L. Ovrebo N. Jay Justice 262 CR 3062 Department of Biology 16055 Michelle Drive Newton, Texas 75966, U.S.A. University of Central Oklahoma Alexander, Arkansas 72002, U.S.A. [email protected] Edmond, Oklahoma 73034, U.S.A. [email protected] [email protected] ABSTRACT This is a second paper documenting the literature records for Texas fleshy basidiomycetous fungi and includes both older literature and recently published papers. We report 80 literature articles which include 14 new taxa described from Texas. We also report on 120 new records of fleshy basdiomycetous fungi collected primarily from southeast Texas. RESUMEN Este es un segundo artículo que documenta el registro de nuevas especies de hongos carnosos basidiomicetos, incluyendo artículos antiguos y recientes. Reportamos 80 artículos científicamente relacionados con estas especies que incluyen 14 taxones con holotipos en Texas. Así mismo, reportamos unos 120 nuevos registros de hongos carnosos basidiomicetos recolectados primordialmente en al sureste de Texas. PART I—MYCOLOGICAL LITERATURE ON TEXAS FLESHY BASIDIOMYCOTA Lewis and Ovrebo (2009) previously reported on literature for Texas fleshy Basidiomycota and also listed new vouchered records for Texas of that group. Presented here is an update to the listing which includes literature published since 2009 and also includes older references that we previously had not uncovered. The authors’ primary research interests center around gilled mushrooms and boletes so perhaps the list that follows is most complete for the fungi of these groups. We have, however, attempted to locate references for all fleshy basidio- mycetous fungi. -

A Preliminary Checklist of Arizona Macrofungi
A PRELIMINARY CHECKLIST OF ARIZONA MACROFUNGI Scott T. Bates School of Life Sciences Arizona State University PO Box 874601 Tempe, AZ 85287-4601 ABSTRACT A checklist of 1290 species of nonlichenized ascomycetaceous, basidiomycetaceous, and zygomycetaceous macrofungi is presented for the state of Arizona. The checklist was compiled from records of Arizona fungi in scientific publications or herbarium databases. Additional records were obtained from a physical search of herbarium specimens in the University of Arizona’s Robert L. Gilbertson Mycological Herbarium and of the author’s personal herbarium. This publication represents the first comprehensive checklist of macrofungi for Arizona. In all probability, the checklist is far from complete as new species await discovery and some of the species listed are in need of taxonomic revision. The data presented here serve as a baseline for future studies related to fungal biodiversity in Arizona and can contribute to state or national inventories of biota. INTRODUCTION Arizona is a state noted for the diversity of its biotic communities (Brown 1994). Boreal forests found at high altitudes, the ‘Sky Islands’ prevalent in the southern parts of the state, and ponderosa pine (Pinus ponderosa P.& C. Lawson) forests that are widespread in Arizona, all provide rich habitats that sustain numerous species of macrofungi. Even xeric biomes, such as desertscrub and semidesert- grasslands, support a unique mycota, which include rare species such as Itajahya galericulata A. Møller (Long & Stouffer 1943b, Fig. 2c). Although checklists for some groups of fungi present in the state have been published previously (e.g., Gilbertson & Budington 1970, Gilbertson et al. 1974, Gilbertson & Bigelow 1998, Fogel & States 2002), this checklist represents the first comprehensive listing of all macrofungi in the kingdom Eumycota (Fungi) that are known from Arizona. -

The Macrofungi Checklist of Liguria (Italy): the Current Status of Surveys
Posted November 2008. Summary published in MYCOTAXON 105: 167–170. 2008. The macrofungi checklist of Liguria (Italy): the current status of surveys MIRCA ZOTTI1*, ALFREDO VIZZINI 2, MIDO TRAVERSO3, FABRIZIO BOCCARDO4, MARIO PAVARINO1 & MAURO GIORGIO MARIOTTI1 *[email protected] 1DIP.TE.RIS - Università di Genova - Polo Botanico “Hanbury”, Corso Dogali 1/M, I16136 Genova, Italy 2 MUT- Università di Torino, Dipartimento di Biologia Vegetale, Viale Mattioli 25, I10125 Torino, Italy 3Via San Marino 111/16, I16127 Genova, Italy 4Via F. Bettini 14/11, I16162 Genova, Italy Abstract— The paper is aimed at integrating and updating the first edition of the checklist of Ligurian macrofungi. Data are related to mycological researches carried out mainly in some holm-oak woods through last three years. The new taxa collected amount to 172: 15 of them belonging to Ascomycota and 157 to Basidiomycota. It should be highlighted that 12 taxa have been recorded for the first time in Italy and many species are considered rare or infrequent. Each taxa reported consists of the following items: Latin name, author, habitat, height, and the WGS-84 Global Position System (GPS) coordinates. This work, together with the original Ligurian checklist, represents a contribution to the national checklist. Key words—mycological flora, new reports Introduction Liguria represents a very interesting region from a mycological point of view: macrofungi, directly and not directly correlated to vegetation, are frequent, abundant and quite well distributed among the species. This topic is faced and discussed in Zotti & Orsino (2001). Observations prove an high level of fungal biodiversity (sometimes called “mycodiversity”) since Liguria, though covering only about 2% of the Italian territory, shows more than 36 % of all the species recorded in Italy. -

BODRUM PAMUKKALE Mark O’Connell Photography 26 102 Istock FETHIYE KAPADOKYA 44 110
TURKEY I MONTENEGRO I GREECE I MIAMI Charter & Brokerage & Charter Yacht Services & Bunkering & Services Yacht Concierge Itinerary Planning Agency Special Events Provisioning www.c2cyacthing.com EXPERIENCE Agency I Concierge I Yacht Services & Bunkering I Provisioning I Itinerary Planning I Special Events I Brokerage I Charter COAST TO COAST SEA TO SEA We enjoy discovering the best places on the most reliable coasts, offering you the best local places, the best entertainment places, the great flavors. Turkey’s most experienced marine service we provide to you for many years. We love the sea and our work. EDITOR CLIFFORD H. POLLEY President & CEO C2C Yachting International Welcome to our latest edition, highlighting We are driven by our mantra to explore not the array of destinations we cover to only what you are going to do, but what you’re spotlighting ideal cruising and vacation not going to do. Allow us to show you. locales. As we navigate the perplexity of life, seize the opportunity to unplug in the serenity From all of us at C2C Yachting we wish you of paradise. every success with your adventures, wherever they may take you. C2C Yachting’s coverage has expanded from Turkey, Greece, Montenegro and Croatia To keep abreast of key information to include our main US office in Miami, please continue to visit our website Florida. Taking our services global allows www.c2cyachting.com us to enhance your experience and the pages which follow exhibit our first-rate capabilities. Sincerely, C2C Yachting takes pride in our commitment to provide the highest level of service inclusive of operational needs, cruising itineraries, dining and nightlife curated to your wishes, all fulfilled by the highest level of professional staff. -

(22:31 Utc) Bodrum/Kos Earthquake and Tsunami; Post Tsunami Field Survey Report
THE 20th JULY 2017 (22:31 UTC) BODRUM/KOS EARTHQUAKE AND TSUNAMI; POST TSUNAMI FIELD SURVEY REPORT Ahmet Cevdet Yalçınera, Alessandro Annunziatob, Gerassimos Papadopoulosc, Gozde Guney Dogana, Hasan Gokhan Gulera, Tarık Eray Cakird, Ceren Ozer Sozdinlere, Ergin Ulutasf, Taro Arikawag, Lutfi Suzenh, Utku Kanoglui, Isikhan Gulera, Pamela Probstb, Costas Synolakisj, aMiddle East Technical University, Department of Civil Engineering, Ocean Engineering Research Center, Turkey bJoint Research Centre, European Commission, Ispra Site, Italy cNational Observatory of Athens, Institute of Geodynamics, Greece dTurkish Chamber of Civil Engineers, Bodrum Branch, Turkey eBogazici University, Department of Geophysics, Kandilli Observatory and Earthquake Research Institute, Turkey fKocaeli University, Department of Geophysical Engineering, Turkey gChuo University, Department of Civil and Environmental Engineering, Japan hMiddle East Technical University, Department of Geological Engineering, Turkey iMiddle East Technical University, Department of Aerospace Engineering, Turkey jUSC, LA, USA, Technical University of Crete, Greece Correspondence: [email protected], [email protected], [email protected] August 17, 2017 (new version) Update History Version 0.0 as preliminary report (containing quick survey results (performed on July 22- 24, 2017) has been publicized on July 28, 2017 which is available at http://users.metu.edu.tr/yalciner/july-21-2017-tsunami-report/Report-Field-Survey-of-tsunami- effects-at-S-of-Bodrum-Peninsula.docx Version -

Gasteroid Mycobiota of Rio Grande Do Sul, Brazil: Tulostomataceae
MYCOTAXON Volume 108, pp. 365–384 April–June 2009 Gasteroid mycobiota of Rio Grande do Sul, Brazil: Tulostomataceae Vagner G. Cortez1, Iuri G. Baseia2 & Rosa Mara B. Silveira1 [email protected], [email protected], [email protected] 1Universidade Federal do Rio Grande do Sul, Departamento de Botânica Av. Bento Gonçalves 9500, 91501-970, Porto Alegre, RS, Brazil 2Universidade Federal do Rio Grande do Norte Departamento de Botânica, Ecologia e Zoologia 59072-970, Natal, RN, Brazil Abstract — The diversity of Tulostomataceae has been investigated in Rio Grande do Sul State in southern Brazil. Eight species belonging to two genera were recognized: Battarrea, represented by B. phalloides, and Tulostoma, represented by T. brasiliense, T. cyclophorum, T. dumeticola, T. exasperatum, T. pygmaeum, T. rickii, and T. striatum. All species are described and illustrated by line drawings and photos, including scanning electron micrographs of the basidiospores. Illustrations of the peridium structure are furnished for most taxa. Key words — Agaricales, gasteromycetes, stalked puffballs Introduction The family Tulostomataceae E. Fisch. (Basidiomycota) comprises stalked puffballs belonging to the genera Battarrea Pers., Battarreoides T. Herrera, Chlamydopus Speg., Queletia Fr., Schizostoma Ehrenb. ex Lév., and Tulostoma Pers. (Kirk et al. 2001). Among these, only Battarrea and Tulostoma have been reported in Brazil, although species of Chlamydopus, Queletia, and Schizostoma are known in Argentina and other neighboring countries (Wright 1949, Mahú 1980, Dios et al. 2004). Tulostoma is the largest genus, with more than 140 species, occurring mainly in xerophilous habitats, and to a lesser extent, in forest environments (Wright 1987). Tulostomataceae was recently included in the Agaricales Underw. -
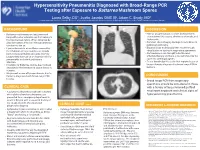
This Poster I It's Designed Large the Placeho Formatted Fo Placeholder
Hypersensitivity Pneumonitis Diagnosed with Broad-Range PCR Testing after Exposure to Battarrea Mushroom Spores • Laura Selby, DO1, Justin Jacobs OMS III2, Adam C. Brady, MD1 Printing: 1. Samaritan Health Services, Corvallis, Oregon 2. Western University of Health Sciences College of Osteopathic Medicine of the Pacific Northwest , Lebanon, Oregon This poster is 48” wide by 36” high. It’s designed to be printed on a BACKGROUND DISCUSSION large • Battarrea mushrooms are long-stemmed • Human lycoperdonosis is a rare disease that is fungi with a characteristic cap that belong to characterized by nausea, shortness of breath, and the Agaricaceae family. When disturbed by tachycardia. physical contact, they can release numerous • Common chest imaging findings include bilateral Customizing the Content: conidia into the air. pulmonary infiltrates. • Lycoperdonosis is a rare illness caused by • Diagnosis has traditionally been made through The placeholders in this the inhalation of large numbers of conidia visualization of spores in respiratory specimens. from certain puffball mushrooms (namely • Corticosteroids are thought to be the most formatted for you. Lycoperdon) that can cause hypersensitivity effective therapy and there is no definitive role for placeholders to add text, or click pneumonitis and mimic pulmonary systemic antifungal agents. infection. • To our knowledge this is the first reported case of an icon to add a table, chart, • Inhalation of Battarrea conidia has not been lycoperdonosis diagnosed by broad range PCR in described in the literature to cause illness in humans. SmartArt graphic, picture or humans. multimedia file. • We present a case of lycoperdonosis due to Battarea, diagnosed with broad-range PCR CONCLUSION T testing. -

Collecting and Recording Fungi
British Mycological Society Recording Network Guidance Notes COLLECTING AND RECORDING FUNGI A revision of the Guide to Recording Fungi previously issued (1994) in the BMS Guides for the Amateur Mycologist series. Edited by Richard Iliffe June 2004 (updated August 2006) © British Mycological Society 2006 Table of contents Foreword 2 Introduction 3 Recording 4 Collecting fungi 4 Access to foray sites and the country code 5 Spore prints 6 Field books 7 Index cards 7 Computers 8 Foray Record Sheets 9 Literature for the identification of fungi 9 Help with identification 9 Drying specimens for a herbarium 10 Taxonomy and nomenclature 12 Recent changes in plant taxonomy 12 Recent changes in fungal taxonomy 13 Orders of fungi 14 Nomenclature 15 Synonymy 16 Morph 16 The spore stages of rust fungi 17 A brief history of fungus recording 19 The BMS Fungal Records Database (BMSFRD) 20 Field definitions 20 Entering records in BMSFRD format 22 Locality 22 Associated organism, substrate and ecosystem 22 Ecosystem descriptors 23 Recommended terms for the substrate field 23 Fungi on dung 24 Examples of database field entries 24 Doubtful identifications 25 MycoRec 25 Recording using other programs 25 Manuscript or typescript records 26 Sending records electronically 26 Saving and back-up 27 Viruses 28 Making data available - Intellectual property rights 28 APPENDICES 1 Other relevant publications 30 2 BMS foray record sheet 31 3 NCC ecosystem codes 32 4 Table of orders of fungi 34 5 Herbaria in UK and Europe 35 6 Help with identification 36 7 Useful contacts 39 8 List of Fungus Recording Groups 40 9 BMS Keys – list of contents 42 10 The BMS website 43 11 Copyright licence form 45 12 Guidelines for field mycologists: the practical interpretation of Section 21 of the Drugs Act 2005 46 1 Foreword In June 2000 the British Mycological Society Recording Network (BMSRN), as it is now known, held its Annual Group Leaders’ Meeting at Littledean, Gloucestershire. -

The 20Th July 2017 (22:31 UTC) Bodrum-Kos Earthquake And
The 20th July 2017 (22:31 UTC) Bodrum-Kos Earthquake and Tsunami; Field Surveys, Lessons and Numerical Modeling Ahmet Cevdet Yalçınera, Alessandro Annunziatob, Gerassimos Papadopoulosc, Gozde Guney Dogana, Hasan Gokhan Gulera, Tarık Eray Cakird, Ceren Ozer Sozdinlere, Ergin Ulutasf, Taro Arikawag, Lutfi Suzenh, Utku Kanoglui, Isikhan Gulera, Pamela Probstb, Costas Synolakisj, aMiddle East Technical University, Department of Civil Engineering, Ocean Engineering Research Center, Turkey; bJoint Research Centre, European Commission, Ispra Site, Italy; cNational Observatory of Athens, Institute of Geodynamics, Greece; dTurkish Chamber of Civil Engineers, Bodrum Branch, Turkey; eBogazici University, Department of Geophysics, Kandilli Observatory and Earthquake Research Institute, Turkey; fKocaeli University, Department of Geophysical Engineering, Turkey; gChuo University, Department of Civil and Environmental Engineering, Japan; hMiddle East Technical University, Department of Geological Engineering, Turkey; iMiddle East Technical University, Department of Aerospace Engineering, Turkey; jUSC, LA, USA, Technical University of Crete, Greece Bodrum and its vicinity Bodrum Gumbet Bay Karaada (Black Island) Fault mechanisms of main shock and aftershocks and Earthquake Intensity Map produced by ELER (KOERI) EARTHQUAKE ACTIVITY BETWEEN 21ST JULY 2017 01:31 - 21ST AUGUST 2017 03:00 (8403 EVENTS) (KOERI) Tsunami measurement at Bodrum tide-gauge station View of sea withdrawal in Bodrum Gumbet Bay about three hours after the earthquake Place Region Lon.