Building Divergent Body Plans with Similar Genetic Pathways
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coastal and Marine Ecological Classification Standard (2012)
FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard Marine and Coastal Spatial Data Subcommittee Federal Geographic Data Committee June, 2012 Federal Geographic Data Committee FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard, June 2012 ______________________________________________________________________________________ CONTENTS PAGE 1. Introduction ..................................................................................................................... 1 1.1 Objectives ................................................................................................................ 1 1.2 Need ......................................................................................................................... 2 1.3 Scope ........................................................................................................................ 2 1.4 Application ............................................................................................................... 3 1.5 Relationship to Previous FGDC Standards .............................................................. 4 1.6 Development Procedures ......................................................................................... 5 1.7 Guiding Principles ................................................................................................... 7 1.7.1 Build a Scientifically Sound Ecological Classification .................................... 7 1.7.2 Meet the Needs of a Wide Range of Users ...................................................... -
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
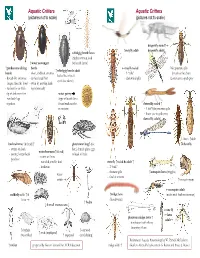
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Mitochondrial Genomes of Two Polydora
www.nature.com/scientificreports OPEN Mitochondrial genomes of two Polydora (Spionidae) species provide further evidence that mitochondrial architecture in the Sedentaria (Annelida) is not conserved Lingtong Ye1*, Tuo Yao1, Jie Lu1, Jingzhe Jiang1 & Changming Bai2 Contrary to the early evidence, which indicated that the mitochondrial architecture in one of the two major annelida clades, Sedentaria, is relatively conserved, a handful of relatively recent studies found evidence that some species exhibit elevated rates of mitochondrial architecture evolution. We sequenced complete mitogenomes belonging to two congeneric shell-boring Spionidae species that cause considerable economic losses in the commercial marine mollusk aquaculture: Polydora brevipalpa and Polydora websteri. The two mitogenomes exhibited very similar architecture. In comparison to other sedentarians, they exhibited some standard features, including all genes encoded on the same strand, uncommon but not unique duplicated trnM gene, as well as a number of unique features. Their comparatively large size (17,673 bp) can be attributed to four non-coding regions larger than 500 bp. We identifed an unusually large (putative) overlap of 14 bases between nad2 and cox1 genes in both species. Importantly, the two species exhibited completely rearranged gene orders in comparison to all other available mitogenomes. Along with Serpulidae and Sabellidae, Polydora is the third identifed sedentarian lineage that exhibits disproportionally elevated rates of mitogenomic architecture rearrangements. Selection analyses indicate that these three lineages also exhibited relaxed purifying selection pressures. Abbreviations NCR Non-coding region PCG Protein-coding gene Metazoan mitochondrial genomes (mitogenomes) usually encode the set of 37 genes, comprising 2 rRNAs, 22 tRNAs, and 13 proteins, encoded on both genomic strands. -

Visceral and Cutaneous Larva Migrans PAUL C
Visceral and Cutaneous Larva Migrans PAUL C. BEAVER, Ph.D. AMONG ANIMALS in general there is a In the development of our concepts of larva II. wide variety of parasitic infections in migrans there have been four major steps. The which larval stages migrate through and some¬ first, of course, was the discovery by Kirby- times later reside in the tissues of the host with¬ Smith and his associates some 30 years ago of out developing into fully mature adults. When nematode larvae in the skin of patients with such parasites are found in human hosts, the creeping eruption in Jacksonville, Fla. (6). infection may be referred to as larva migrans This was followed immediately by experi¬ although definition of this term is becoming mental proof by numerous workers that the increasingly difficult. The organisms impli¬ larvae of A. braziliense readily penetrate the cated in infections of this type include certain human skin and produce severe, typical creep¬ species of arthropods, flatworms, and nema¬ ing eruption. todes, but more especially the nematodes. From a practical point of view these demon¬ As generally used, the term larva migrans strations were perhaps too conclusive in that refers particularly to the migration of dog and they encouraged the impression that A. brazil¬ cat hookworm larvae in the human skin (cu¬ iense was the only cause of creeping eruption, taneous larva migrans or creeping eruption) and detracted from equally conclusive demon¬ and the migration of dog and cat ascarids in strations that other species of nematode larvae the viscera (visceral larva migrans). In a still have the ability to produce similarly the pro¬ more restricted sense, the terms cutaneous larva gressive linear lesions characteristic of creep¬ migrans and visceral larva migrans are some¬ ing eruption. -

Animal Phylum Poster Porifera
Phylum PORIFERA CNIDARIA PLATYHELMINTHES ANNELIDA MOLLUSCA ECHINODERMATA ARTHROPODA CHORDATA Hexactinellida -- glass (siliceous) Anthozoa -- corals and sea Turbellaria -- free-living or symbiotic Polychaetes -- segmented Gastopods -- snails and slugs Asteroidea -- starfish Trilobitomorpha -- tribolites (extinct) Urochordata -- tunicates Groups sponges anemones flatworms (Dugusia) bristleworms Bivalves -- clams, scallops, mussels Echinoidea -- sea urchins, sand Chelicerata Cephalochordata -- lancelets (organisms studied in detail in Demospongia -- spongin or Hydrazoa -- hydras, some corals Trematoda -- flukes (parasitic) Oligochaetes -- earthworms (Lumbricus) Cephalopods -- squid, octopus, dollars Arachnida -- spiders, scorpions Mixini -- hagfish siliceous sponges Xiphosura -- horseshoe crabs Bio1AL are underlined) Cubozoa -- box jellyfish, sea wasps Cestoda -- tapeworms (parasitic) Hirudinea -- leeches nautilus Holothuroidea -- sea cucumbers Petromyzontida -- lamprey Mandibulata Calcarea -- calcareous sponges Scyphozoa -- jellyfish, sea nettles Monogenea -- parasitic flatworms Polyplacophora -- chitons Ophiuroidea -- brittle stars Chondrichtyes -- sharks, skates Crustacea -- crustaceans (shrimp, crayfish Scleropongiae -- coralline or Crinoidea -- sea lily, feather stars Actinipterygia -- ray-finned fish tropical reef sponges Hexapoda -- insects (cockroach, fruit fly) Sarcopterygia -- lobed-finned fish Myriapoda Amphibia (frog, newt) Chilopoda -- centipedes Diplopoda -- millipedes Reptilia (snake, turtle) Aves (chicken, hummingbird) Mammalia -

Evolution of Direct-Developing Larvae: Selection Vs Loss Margaret Snoke Smith,1 Kirk S
Hypotheses Evolution of direct-developing larvae: selection vs loss Margaret Snoke Smith,1 Kirk S. Zigler,2 and Rudolf A. Raff1,3* Summary echinoderms, sea urchins, reveal that a majority of sea urchin Observations of a sea urchin larvae show that most species exhibit one of two life history strategies (Fig. 1).(10) species adopt one of two life history strategies. One strategy is to make numerous small eggs, which develop One strategy is to make many small eggs that develop into a into a larva with a required feeding period in the water larva that must feed and grow for weeks or months in the water column before metamorphosis. In contrast, the second column before metamorphosis. In the water column, these strategy is to make fewer large eggs with a larva that does larvae are dependent on plankton abundance for food and are not feed, which reduces the time to metamorphosis and subject to high levels of predation.(11) The other strategy thus the time spent in the water column. The larvae associated with each strategy have distinct morpholo- involves increasing egg size and maternal provisioning, which gies and developmental processes that reflect their decreases the reliance on planktonic food sources and the feeding requirements, so that those that feed exhibit time to metamorphosis, thus minimizing larval mortality. indirect development with a complex larva, and those that However, assuming finite resources for egg production, do not feed form a morphologically simplified larva and increasing egg size also reduces the total number of eggs exhibit direct development. Phylogenetic studies show that, in sea urchins, a feeding larva, the pluteus, is the produced. -

Hookworm-Related Cutaneous Larva Migrans
326 Hookworm-Related Cutaneous Larva Migrans Patrick Hochedez , MD , and Eric Caumes , MD Département des Maladies Infectieuses et Tropicales, Hôpital Pitié-Salpêtrière, Paris, France DOI: 10.1111/j.1708-8305.2007.00148.x Downloaded from https://academic.oup.com/jtm/article/14/5/326/1808671 by guest on 27 September 2021 utaneous larva migrans (CLM) is the most fre- Risk factors for developing HrCLM have specifi - Cquent travel-associated skin disease of tropical cally been investigated in one outbreak in Canadian origin. 1,2 This dermatosis fi rst described as CLM by tourists: less frequent use of protective footwear Lee in 1874 was later attributed to the subcutane- while walking on the beach was signifi cantly associ- ous migration of Ancylostoma larvae by White and ated with a higher risk of developing the disease, Dove in 1929. 3,4 Since then, this skin disease has also with a risk ratio of 4. Moreover, affected patients been called creeping eruption, creeping verminous were somewhat younger than unaffected travelers dermatitis, sand worm eruption, or plumber ’ s itch, (36.9 vs 41.2 yr, p = 0.014). There was no correla- which adds to the confusion. It has been suggested tion between the reported amount of time spent on to name this disease hookworm-related cutaneous the beach and the risk of developing CLM. Consid- larva migrans (HrCLM).5 ering animals in the neighborhood, 90% of the Although frequent, this tropical dermatosis is travelers in that study reported seeing cats on the not suffi ciently well known by Western physicians, beach and around the hotel area, and only 1.5% and this can delay diagnosis and effective treatment. -

An Anatomical Description of a Miniaturized Acorn Worm (Hemichordata, Enteropneusta) with Asexual Reproduction by Paratomy
An Anatomical Description of a Miniaturized Acorn Worm (Hemichordata, Enteropneusta) with Asexual Reproduction by Paratomy The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Worsaae, Katrine, Wolfgang Sterrer, Sabrina Kaul-Strehlow, Anders Hay-Schmidt, and Gonzalo Giribet. 2012. An anatomical description of a miniaturized acorn worm (hemichordata, enteropneusta) with asexual reproduction by paratomy. PLoS ONE 7(11): e48529. Published Version doi:10.1371/journal.pone.0048529 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:11732117 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA An Anatomical Description of a Miniaturized Acorn Worm (Hemichordata, Enteropneusta) with Asexual Reproduction by Paratomy Katrine Worsaae1*, Wolfgang Sterrer2, Sabrina Kaul-Strehlow3, Anders Hay-Schmidt4, Gonzalo Giribet5 1 Marine Biological Section, Department of Biology, University of Copenhagen, Copenhagen, Denmark, 2 Bermuda Natural History Museum, Flatts, Bermuda, 3 Department for Molecular Evolution and Development, University of Vienna, Vienna, Austria, 4 Department of Neuroscience and Pharmacology, The Panum Institute, University of Copenhagen, Copenhagen, Denmark, 5 Museum of Comparative Zoology, Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachussetts, United States of America Abstract The interstitial environment of marine sandy bottoms is a nutrient-rich, sheltered habitat whilst at the same time also often a turbulent, space-limited, and ecologically challenging environment dominated by meiofauna. The interstitial fauna is one of the most diverse on earth and accommodates miniaturized representatives from many macrofaunal groups as well as several exclusively meiofaunal phyla. -

Evolutionary Developmental Biology 573
EVOC20 29/08/2003 11:15 AM Page 572 Evolutionary 20 Developmental Biology volutionary developmental biology, now often known Eas “evo-devo,” is the study of the relation between evolution and development. The relation between evolution and development has been the subject of research for many years, and the chapter begins by looking at some classic ideas. However, the subject has been transformed in recent years as the genes that control development have begun to be identified. This chapter looks at how changes in these developmental genes, such as changes in their spatial or temporal expression in the embryo, are associated with changes in adult morphology. The origin of a set of genes controlling development may have opened up new and more flexible ways in which evolution could occur: life may have become more “evolvable.” EVOC20 29/08/2003 11:15 AM Page 573 CHAPTER 20 / Evolutionary Developmental Biology 573 20.1 Changes in development, and the genes controlling development, underlie morphological evolution Morphological structures, such as heads, legs, and tails, are produced in each individual organism by development. The organism begins life as a single cell. The organism grows by cell division, and the various cell types (bone cells, skin cells, and so on) are produced by differentiation within dividing cell lines. When one species evolves into Morphological evolution is driven another, with a changed morphological form, the developmental process must have by developmental evolution changed too. If the descendant species has longer legs, it is because the developmental process that produces legs has been accelerated, or extended over time. -

Hemichordate Phylogeny: a Molecular, and Genomic Approach By
Hemichordate Phylogeny: A molecular, and genomic approach by Johanna Taylor Cannon A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama May 4, 2014 Keywords: phylogeny, evolution, Hemichordata, bioinformatics, invertebrates Copyright 2014 by Johanna Taylor Cannon Approved by Kenneth M. Halanych, Chair, Professor of Biological Sciences Jason Bond, Associate Professor of Biological Sciences Leslie Goertzen, Associate Professor of Biological Sciences Scott Santos, Associate Professor of Biological Sciences Abstract The phylogenetic relationships within Hemichordata are significant for understanding the evolution of the deuterostomes. Hemichordates possess several important morphological structures in common with chordates, and they have been fixtures in hypotheses on chordate origins for over 100 years. However, current evidence points to a sister relationship between echinoderms and hemichordates, indicating that these chordate-like features were likely present in the last common ancestor of these groups. Therefore, Hemichordata should be highly informative for studying deuterostome character evolution. Despite their importance for understanding the evolution of chordate-like morphological and developmental features, relationships within hemichordates have been poorly studied. At present, Hemichordata is divided into two classes, the solitary, free-living enteropneust worms, and the colonial, tube- dwelling Pterobranchia. The objective of this dissertation is to elucidate the evolutionary relationships of Hemichordata using multiple datasets. Chapter 1 provides an introduction to Hemichordata and outlines the objectives for the dissertation research. Chapter 2 presents a molecular phylogeny of hemichordates based on nuclear ribosomal 18S rDNA and two mitochondrial genes. In this chapter, we suggest that deep-sea family Saxipendiidae is nested within Harrimaniidae, and Torquaratoridae is affiliated with Ptychoderidae. -

Insect Life Cycle ���������������������������������� a Reading A–Z Level L Leveled Book Word Count: 607
Insect Life Cycle A Reading A–Z Level L Leveled Book Word Count: 607 Written by Chuck Garofano Visit www.readinga-z.com www.readinga-z.com for thousands of books and materials. Photo Credits: Front cover, back cover, pages 3, 4, 7, 13, 15 (top): © Brand X Pictures; title page, page 11: © Kenneth Keifer/123RF; page 5: © Eric Isselée/ Dreamstime.com; page 6: © Mike Abbey/Visuals Unlimited, Inc.; page 8: © Richard Williams/123RF; page 9: © Oxford Scientific/Peter Arnold; page 10: © Anthony Bannister/Gallo Images/Corbis; page 12: © Dennis Johnson; Papillo/Corbis; page 14: © 123RF; page 15 (bottom): © ArtToday Written by Chuck Garofano Insect Life Cycle Level L Leveled Book Correlation © Learning A–Z LEVEL L Written by Chuck Garofano Fountas & Pinnell K All rights reserved. Reading Recovery 18 www.readinga-z.com www.readinga-z.com DRA 20 Table of Contents Flower beetle Introduction ................ 4 What Are Insects? ............ 6 Egg ....................... 8 Larva ......................10 Pupa ..................... 12 Ladybug Tropical ant Adult ..................... 13 Introduction Nymph . 14 When you were born, your body Index...................... 16 was shaped a lot like it is now. It was smaller, of course, but you had a head, legs, arms, and a torso. When you grow up, your body shape will be about the same. But some baby animals look nothing like the adults they will become. Insect Life Cycle • Level L 3 4 These animals have a different kind What Are Insects? of life cycle. A life cycle is the series There are more than 800,000 of changes an animal goes through different kinds of insects. -

Information to Users
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter ^ce, while others may t>e from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy subm itted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will t>e noted. Also, if unauthorized copyright material had to t>e removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, t>eginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. Photographs included in ttie original manuscript have been reproduced xerographically in this copy. Higher quality 6” x 9” black arxt white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. Bell & Howell Information and Learning 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA 800-521-0600 UMI* Phylogeny of Vestimentiferan Tube Worms by Anja Schulze Diplom, University of Bielefeld, 1995 A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY in the Department of Biology We accept this dissertation as conforming to the required standard Dr.