Development of a Lecithotrophic Pilidium Larva Illustrates Convergent Evolution of Trochophore-Like Morphology Marie K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The A/P Axis in Echinoderm Ontogeny and Evolution: Evidence from Fossils and Molecules
EVOLUTION & DEVELOPMENT 2:2, 93–101 (2000) The A/P axis in echinoderm ontogeny and evolution: evidence from fossils and molecules Kevin J. Peterson,a,b César Arenas-Mena,a,c and Eric H. Davidsona,* aDivision of Biology, California Institute of Technology, Pasadena, CA 91125, USA; bDivision of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 91125, USA; cStowers Institute for Medical Research, Kansas City, MO 64110, USA *Author for correspondence (email: [email protected]) SUMMARY Even though echinoderms are members of the such that there is but a single plane of symmetry dividing the Bilateria, the location of their anterior/posterior axis has re- animal into left and right halves. We tentatively hypothesize mained enigmatic. Here we propose a novel solution to the that this plane of symmetry is positioned along the dorsal/ven- problem employing three lines of evidence: the expression of tral axis. These axis identifications lead to the conclusion that a posterior class Hox gene in the coeloms of the nascent the five ambulacra are not primary body axes, but instead are adult body plan within the larva; the anatomy of certain early outgrowths from the central anterior/posterior axis. These fossil echinoderms; and finally the relation between endo- identifications also shed insight into several other evolutionary skeletal plate morphology and the associated coelomic tis- mysteries of various echinoderm clades such as the indepen- sues. All three lines of evidence converge on the same answer, dent evolution of bilateral symmetry in irregular echinoids, but namely that the location of the adult mouth is anterior, and the do not elucidate the underlying mechanisms of the adult co- anterior/posterior axis runs from the mouth through the adult elomic architecture. -

Classification
Science Classification Pupil Workbook Year 5 Unit 5 Name: 2 3 Existing Knowledge: Why do we put living things into different groups and what are the groups that we can separate them into? You can think about the animals in the picture and all the others that you know. 4 Session 1: How do we classify animals with a backbone? Key Knowledge Key Vocabulary Animals known as vertebrates have a spinal column. Vertebrates Some vertebrates are warm-blooded meaning that they Species maintain a consistent body temperature. Some are cold- Habitat blooded, meaning they need to move around to warm up or cool down. Spinal column Vertebrates are split into five main groups known as Warm-blooded/Cold- mammals, amphibians, reptiles, birds and fish. blooded Task: Look at the picture here and think about the different groups that each animal is part of. How is each different to the others and which other animals share similar characteristics? Write your ideas here: __________________________ __________________________ __________________________ __________________________ __________________________ __________________________ ____________________________________________________ ____________________________________________________ ____________________________________________________ ____________________________________________________ ____________________________________________________ 5 How do we classify animals with a backbone? Vertebrates are the most advanced organisms on Earth. The traits that make all of the animals in this group special are -

Amphipholis Squamata MICHAEL P
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Aug. 1990, p. 2436-2440 Vol. 56, No. 8 0099-2240/90/082436-05$02.00/0 Copyright C) 1990, American Society for Microbiology Description of a Novel Symbiotic Bacterium from the Brittle Star, Amphipholis squamata MICHAEL P. LESSERt* AND RICHARD P. BLAKEMORE Department of Microbiology, University of New Hampshire, Durham, New Hampshire 03824 Received 8 November 1989/Accepted 3 June 1990 A gram-negative, marine, facultatively anaerobic bacterial isolate designated strain AS-1 was isolated from the subcuticular space of the brittle star, Amphipholis squamata. Its sensitivity to 0/129 and novobiocin, overall morphology, and biochemical characteristics and the moles percent guanine-plus-cytosine composition of its DNA (42.9 to 44.4) suggest that this isolate should be placed in the genus Vibrio. Strain AS-1 was not isolated from ambient seawater and is distinct from described Vibrio species. This symbiotic bacterium may assist its host as one of several mechanisms of nutrient acquisition during the brooding of developing embryos. The biology of bacterium-invertebrate symbiotic associa- isopropyl alcohol for 30 s and two rinses in sterile ASW. tions has elicited considerable interest, particularly since the Logarithmic dilutions were plated on Zobell modified 2216E discoveries during the past decade of chemoautotrophic medium (ASW, 1 g of peptone liter-1, 1 g of yeast extract symbiotic bacteria associated with several invertebrate spe- liter-' [pH 7.8 to 8.4]) (29), as were samples of ambient cies in sulfide-rich habitats (4, 5). Bacterial-invertebrate seawater from the site of collection and ASW controls. All symbioses (mutualistic) have been reported from many materials and equipment were sterilized, and all procedures invertebrate taxa, examples of which include cellulolytic were performed by aseptic techniques. -

Fauna Ryukyuana ISSN 2187-6657
Fauna Ryukyuana ISSN 2187-6657 http://w3.u-ryukyu.ac.jp/naruse/lab/Fauna_Ryukyuana.html Records of Notospermus tricuspidatus (Quoy & Gaimard, 1833) (Nemertea: Pilidiophora) in Japanese waters, with a review of warm-water green-bodied heteronemerteans from the Indo–West-Pacific Hiroshi Kajihara1, Ira Hosokawa2, 3, Koji Hosokawa3 & Ryuta Yoshida4 1Faculty of Science, Hokkaido University, N10W8, Kita-ku, Sapporo 060-0810, Japan (e-mail: [email protected]) 2Kamiyama Elementary School, Hara 3-1, Yakushima, Kumage 891-4403, Kagoshima, Japan 3Mugio 318-9, Yakushima, Kumage 891-4402, Kagoshima, Japan 4Iriomote Station, Tropical Biosphere Research Centre, University of the Ryukyus, Uehara 870, Taketomi, Yaeyama 907-1541, Okinawa, Japan Abstract. The heteronemertean Notospermus Actually, however, no nemertean with a zigzag tricuspidatus (Quoy & Gaimard, 1833) [new marking has ever been reported from Japanese Japanese name: mitsuyari-midori-himomushi] is waters, meaning that there is no formal record of N. known to be distributed in the tropical Indo–West- tricuspidatus in Japan if it is a different species from Pacific, but has not been formally reported from L. albovittatus (Kajihara 2007). Whether or not these Japanese waters based on voucher material. We two forms belong to a single biological entity should summarize records of the species based on recently be tested in future molecular studies, but we collected specimens in the Nansei Islands. A tentatively regard N. tricuspidatus s.str. as specimen collected on Yakushima Island (ca. 30°N) represented by individuals with a zigzag marking, represents the northern limit of the species’ while L. albovittatus as having a straight band. distribution. -
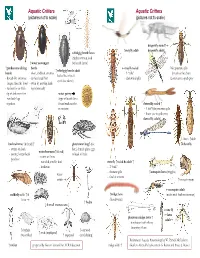
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Redescription of Micrura Dellechiajei (Hubrecht, 1879) (Nemertea
Journal of the Marine Biological Association of the United Kingdom, page 1 of 10. # Marine Biological Association of the United Kingdom, 2015 doi:10.1017/S0025315415000090 Redescription of Micrura dellechiajei (Hubrecht, 1879) (Nemertea, Pilidiophora, Lineidae), a rare Mediterranean species alfonso herrera-bachiller1, sebastian kvist2, gonzalo giribet2 and juan junoy1 1EU-US Marine Biodiversity Research Group, Instituto Franklin, Universidad de Alcala´ & Departamento de Ciencias de la Vida, Universidad de Alcala´, 28871 Alcala´ de Henares, Madrid, Spain, 2Museum of Comparative Zoology, Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, USA The heteronemertean species Micrura dellechiajei is thus far only known from its type locality in the Gulf of Naples (Italy) and has not been recorded in 120 years. During two oceanographic surveys conducted in Spanish Mediterranean waters, several nemertean specimens were collected, and thorough morphological examination indicated that some of these pertained to the species M. dellechiajei, suggesting that populations may be more widespread than previously thought. Because of the rarity of this species coupled with the fact that its last morphological narrative was given 120 years ago, we here provide a redescription of the species based on the new specimens, complete with illustrations and new data concerning its morphology, and we also place some of the collected specimens in a molecular phylogenetic framework. Keywords: Heteronemertea, Pilidiophora, -

Phylum Nemertea)
THE BIOLOGY AND SYSTEMATICS OF A NEW SPECIES OF RIBBON WORM, GENUS TUBULANUS (PHYLUM NEMERTEA) By Rebecca Kirk Ritger Submitted to the Faculty of the College of Arts and Sciences of American University in Partial Fulfillment of the Requirements for the Degree of Master of Science In Biology Chair: Dr. Qiristopher'Tudge m Dr.David C r. Jon L. Norenburg Dean of the College of Arts and Sciences JuK4£ __________ Date 2004 American University Washington, D.C. 20016 AMERICAN UNIVERSITY LIBRARY 1 1 0 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 1421360 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. ® UMI UMI Microform 1421360 Copyright 2004 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, Ml 48106-1346 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. THE BIOLOGY AND SYSTEMATICS OF A NEW SPECIES OF RIBBON WORM, GENUS TUBULANUS (PHYLUM NEMERTEA) By Rebecca Kirk Ritger ABSTRACT Most nemerteans are studied from poorly preserved museum specimens. -

Visceral and Cutaneous Larva Migrans PAUL C
Visceral and Cutaneous Larva Migrans PAUL C. BEAVER, Ph.D. AMONG ANIMALS in general there is a In the development of our concepts of larva II. wide variety of parasitic infections in migrans there have been four major steps. The which larval stages migrate through and some¬ first, of course, was the discovery by Kirby- times later reside in the tissues of the host with¬ Smith and his associates some 30 years ago of out developing into fully mature adults. When nematode larvae in the skin of patients with such parasites are found in human hosts, the creeping eruption in Jacksonville, Fla. (6). infection may be referred to as larva migrans This was followed immediately by experi¬ although definition of this term is becoming mental proof by numerous workers that the increasingly difficult. The organisms impli¬ larvae of A. braziliense readily penetrate the cated in infections of this type include certain human skin and produce severe, typical creep¬ species of arthropods, flatworms, and nema¬ ing eruption. todes, but more especially the nematodes. From a practical point of view these demon¬ As generally used, the term larva migrans strations were perhaps too conclusive in that refers particularly to the migration of dog and they encouraged the impression that A. brazil¬ cat hookworm larvae in the human skin (cu¬ iense was the only cause of creeping eruption, taneous larva migrans or creeping eruption) and detracted from equally conclusive demon¬ and the migration of dog and cat ascarids in strations that other species of nematode larvae the viscera (visceral larva migrans). In a still have the ability to produce similarly the pro¬ more restricted sense, the terms cutaneous larva gressive linear lesions characteristic of creep¬ migrans and visceral larva migrans are some¬ ing eruption. -

Biology of Echinoderms
Echinoderms Branches on the Tree of Life Programs ECHINODERMS Written and photographed by David Denning and Bruce Russell Produced by BioMEDIA ASSOCIATES ©2005 - Running time 16 minutes. Order Toll Free (877) 661-5355 Order by FAX (843) 470-0237 The Phylum Echinodermata consists of about 6,000 living species, all of which are marine. This video program compares the five major classes of living echinoderms in terms of basic functional biology, evolution and ecology using living examples, animations and a few fossil species. Detailed micro- and macro- photography reveal special adaptations of echinoderms and their larval biology. (THUMBNAIL IMAGES IN THIS GUIDE ARE FROM THE VIDEO PROGRAM) Summary of the Program: Introduction - Characteristics of the Class Echinoidea phylum. spine adaptations, pedicellaria, Aristotle‘s lantern, sand dollars, urchin development, Class Asteroidea gastrulation, settlement skeleton, water vascular system, tube feet function, feeding, digestion, Class Holuthuroidea spawning, larval development, diversity symmetry, water vascular system, ossicles, defensive mechanisms, diversity, ecology Class Ophiuroidea regeneration, feeding, diversity Class Crinoidea – Topics ecology, diversity, fossil echinoderms © BioMEDIA ASSOCIATES (1 of 7) Echinoderms ... ... The characteristics that distinguish Phylum Echinodermata are: radial symmetry, internal skeleton, and water-vascular system. Echinoderms appear to be quite different than other ‘advanced’ animal phyla, having radial (spokes of a wheel) symmetry as adults, rather than bilateral (worm-like) symmetry as in other triploblastic (three cell-layer) animals. Viewers of this program will observe that echinoderm radial symmetry is secondary; echinoderms begin as bilateral free-swimming larvae and become radial at the time of metamorphosis. Also, in one echinoderm group, the sea cucumbers, partial bilateral symmetry is retained in the adult stages -- sea cucumbers are somewhat worm–like. -

A Phylum-Wide Survey Reveals Multiple Independent Gains of Head Regeneration Ability in Nemertea
bioRxiv preprint doi: https://doi.org/10.1101/439497; this version posted October 11, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. A phylum-wide survey reveals multiple independent gains of head regeneration ability in Nemertea Eduardo E. Zattara1,2,5, Fernando A. Fernández-Álvarez3, Terra C. Hiebert4, Alexandra E. Bely2 and Jon L. Norenburg1 1 Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA 2 Department of Biology, University of Maryland, College Park, MD, USA 3 Institut de Ciències del Mar, Consejo Superior de Investigaciones Científicas, Barcelona, Spain 4 Institute of Ecology and Evolution, University of Oregon, Eugene, OR, USA 5 INIBIOMA, Consejo Nacional de Investigaciones Científicas y Tecnológicas, Bariloche, RN, Argentina Corresponding author: E.E. Zattara, [email protected] Abstract Animals vary widely in their ability to regenerate, suggesting that regenerative abilities have a rich evolutionary history. However, our understanding of this history remains limited because regeneration ability has only been evaluated in a tiny fraction of species. Available comparative regeneration studies have identified losses of regenerative ability, yet clear documentation of gains is lacking. We surveyed regenerative ability in 34 species spanning the phylum Nemertea, assessing the ability to regenerate heads and tails either through our own experiments or from literature reports. Our sampling included representatives of the 10 most diverse families and all three orders comprising this phylum. -

Evolution of Direct-Developing Larvae: Selection Vs Loss Margaret Snoke Smith,1 Kirk S
Hypotheses Evolution of direct-developing larvae: selection vs loss Margaret Snoke Smith,1 Kirk S. Zigler,2 and Rudolf A. Raff1,3* Summary echinoderms, sea urchins, reveal that a majority of sea urchin Observations of a sea urchin larvae show that most species exhibit one of two life history strategies (Fig. 1).(10) species adopt one of two life history strategies. One strategy is to make numerous small eggs, which develop One strategy is to make many small eggs that develop into a into a larva with a required feeding period in the water larva that must feed and grow for weeks or months in the water column before metamorphosis. In contrast, the second column before metamorphosis. In the water column, these strategy is to make fewer large eggs with a larva that does larvae are dependent on plankton abundance for food and are not feed, which reduces the time to metamorphosis and subject to high levels of predation.(11) The other strategy thus the time spent in the water column. The larvae associated with each strategy have distinct morpholo- involves increasing egg size and maternal provisioning, which gies and developmental processes that reflect their decreases the reliance on planktonic food sources and the feeding requirements, so that those that feed exhibit time to metamorphosis, thus minimizing larval mortality. indirect development with a complex larva, and those that However, assuming finite resources for egg production, do not feed form a morphologically simplified larva and increasing egg size also reduces the total number of eggs exhibit direct development. Phylogenetic studies show that, in sea urchins, a feeding larva, the pluteus, is the produced. -

Title Records of Notospermus Tricuspidatus
Records of Notospermus tricuspidatus (Quoy & Gaimard, 1833) (Nemertea: Pilidiophora) in Japanese waters, with a Title review of warm-water green-bodied heteronemerteans from the Indo‒West-Pacific Kajihara, Hiroshi; Hasokawa, Ira; Hosokawa, Koji; Yoshida, Author(s) Ryuta Citation Fauna Ryukyuana, 28: 59-65 Issue Date 2016-02-03 URL http://hdl.handle.net/20.500.12000/38720 Rights Fauna Ryukyuana ISSN 2187-6657 http://w3.u-ryukyu.ac.jp/naruse/lab/Fauna_Ryukyuana.html Records of Notospermus tricuspidatus (Quoy & Gaimard, 1833) (Nemertea: Pilidiophora) in Japanese waters, with a review of warm-water green-bodied heteronemerteans from the Indo–West-Pacific Hiroshi Kajihara1, Ira Hosokawa2, 3, Koji Hosokawa3 & Ryuta Yoshida4 1Faculty of Science, Hokkaido University, N10W8, Kita-ku, Sapporo 060-0810, Japan (e-mail: [email protected]) 2Kamiyama Elementary School, Hara 3-1, Yakushima, Kumage 891-4403, Kagoshima, Japan 3Mugio 318-9, Yakushima, Kumage 891-4402, Kagoshima, Japan 4Iriomote Station, Tropical Biosphere Research Centre, University of the Ryukyus, Uehara 870, Taketomi, Yaeyama 907-1541, Okinawa, Japan Abstract. The heteronemertean Notospermus Actually, however, no nemertean with a zigzag tricuspidatus (Quoy & Gaimard, 1833) [new marking has ever been reported from Japanese Japanese name: mitsuyari-midori-himomushi] is waters, meaning that there is no formal record of N. known to be distributed in the tropical Indo–West- tricuspidatus in Japan if it is a different species from Pacific, but has not been formally reported from L. albovittatus (Kajihara 2007). Whether or not these Japanese waters based on voucher material. We two forms belong to a single biological entity should summarize records of the species based on recently be tested in future molecular studies, but we collected specimens in the Nansei Islands.