Evolutionary Developmental Biology 573
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AP Biology Planner
COURSE PLANNER Please note that not all activities for the course are listed, and many activities will be supplemented with additional activities such as diagram analysis, practice essays, etc. Intro to AP Biology/Scientific Method This course begins with introducing students to the seven science practices, in addition to the Big Ideas that unify the course. Students complete the lab exercises that emphasize the need for controls in experiments as well as to identify and explain potential sources of error as part of the normal scientific process. Reading in Campbell and Reece: Topics: • Intro to AP biology, the test and how to study. ♣ Activity: Theme-teams: Students research and present scientist who represent each Big Idea in AP Biology. ♣ Activity: Analyzing the AP multiple choice questions with diagrams. • Scientific Method: Steps and controlled experiments ♣ Activity: What is Science? Discussion/Poster Creation. ♣ Activity: Lab Safety activity: NIH’s Student Lab Safety Introduction Course. https://www.safetytraining.nih.gov/introcourse/labsafetyIntro.aspx ♣ Activity: Identify risks in procedures and materials (MSDS analysis) ♣ Activity: Guide to good graphing. ♣ Activity: Examining bias in science. ♣ Lab: Student Designed inquiry. The effect of environmental factors on the metabolic rate of yeast. In this student designed inquiry, students investigate environmental factors as they relate to CO2 production or O2 consumption in yeast. Students must select an independent variable and dependent variable, as well as design an experimental procedure based on an existing lab protocol. ECOLOGY The ecology unit introduces the ideas of energy processes and interactions, which are the focus of Big Idea 2 and 4. Ecology introduces students to creating, analyzing, and evaluating mathematical models in biology and prepares students to tackle evolution, the central unit in AP Biology. -

Role of Hox Genes in Regulating Digit Patterning ROCÍO PÉREZ-GÓMEZ, ENDIKA HARO, MARC FERNÁNDEZ-GUERRERO, MARÍA F
Int. J. Dev. Biol. 62: 797-805 (2018) https://doi.org/10.1387/ijdb.180200mr www.intjdevbiol.com Role of Hox genes in regulating digit patterning ROCÍO PÉREZ-GÓMEZ, ENDIKA HARO, MARC FERNÁNDEZ-GUERRERO, MARÍA F. BASTIDA and MARÍA A. ROS* Instituto de Biomedicina y Biotecnología de Cantabria, CSIC–SODERCAN Universidad de Cantabria, Santander, Spain ABSTRACT The distal part of the tetrapod limb, the autopod, is characterized by the presence of digits. The digits display a wide diversity of shapes and number reflecting selection pressure for functional adaptation. Despite extensive study, the different aspects of digit patterning, as well as the factors and mechanisms involved are not completely understood. Here, we review the evidence implicating Hox proteins in digit patterning and the interaction between Hox genes and the Sonic hedgehog/Gli3 pathway, the other major regulator of digit number and identity. Currently, it is well accepted that a self-organizing Turing-type mechanism underlies digit patterning, this being understood as the establishment of an iterative arrangement of digit/interdigit in the hand plate. We also discuss the involvement of 5’ Hox genes in regulating digit spacing in the digital plate and therefore the number of digits formed in this self-organizing system. KEY WORDS: limb development, Hox gene, digit patterning, Shh, Gli3 Introduction and Meyer, 2015). The digits are crucial elements for the function of the limb. They The basic plan of the tetrapod limb includes three distinct can be viewed as serial identical structures arranged along the proximo-distal (PD) segments: the stylopod (arm), the zeugopod antero-posterior (AP) axis of the autopod, thumb to little finger, or (forearm) and the autopod (hand/foot). -

Review Heterochronic Genes and the Nature of Developmental Time
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology 17, R425–R434, June 5, 2007 ª2007 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2007.03.043 Heterochronic Genes and the Nature of Review Developmental Time Eric G. Moss that have arisen to solve the problem of regulated tim- ing in animal development. Timing is a fundamental issue in development, with Heterochrony and Developmental Timing a range of implications from birth defects to evolu- in Evolution tion. In the roundworm Caenorhabditis elegans, Changes in developmental timing have long been be- the heterochronic genes encode components of lieved to be a major force in the evolution of morphol- a molecular developmental timing mechanism. This ogy [1]. A variety of changes is encompassed by the mechanism functions in diverse cell types through- concept of ‘heterochrony’ — differences in the relative out the animal to specify cell fates at each larval timing of developmental events between two closely stage. MicroRNAs play an important role in this related species. A classic example of heterochrony is mechanism by stage-specifically repressing cell- the axolotl. This salamander reaches sexual maturity fate regulators. Recent studies reveal the surprising without undergoing metamorphosis, such that its complexity surrounding this regulation — for exam- non-gonadal tissues retain larval features of other ple, a positive feedback loop may make the regula- salamanders. Different species of axolotls exhibit ge- tion more robust, and certain components of the netic differences in the production or activity of thyroid mechanism are expressed in brief periods at each hormones that trigger metamorphosis from aquatic stage. -

How Morphological Development Can Guide Evolution Sam Kriegman1,*, Nick Cheney1, and Josh Bongard1
How morphological development can guide evolution Sam Kriegman1,*, Nick Cheney1, and Josh Bongard1 1University of Vermont, Department of Computer Science, Burlington, VT, USA *[email protected] ABSTRACT Organisms result from adaptive processes interacting across different time scales. One such interaction is that between development and evolution. Models have shown that development sweeps over several traits in a single agent, sometimes exposing promising static traits. Subsequent evolution can then canalize these rare traits. Thus, development can, under the right conditions, increase evolvability. Here, we report on a previously unknown phenomenon when embodied agents are allowed to develop and evolve: Evolution discovers body plans robust to control changes, these body plans become genetically assimilated, yet controllers for these agents are not assimilated. This allows evolution to continue climbing fitness gradients by tinkering with the developmental programs for controllers within these permissive body plans. This exposes a previously unknown detail about the Baldwin effect: instead of all useful traits becoming genetically assimilated, only traits that render the agent robust to changes in other traits become assimilated. We refer to this as differential canalization. This finding also has implications for the evolutionary design of artificial and embodied agents such as robots: robots robust to internal changes in their controllers may also be robust to external changes in their environment, such as transferal from simulation to reality or deployment in novel environments. Introduction The shape of life changes on many different time scales. From generation to generation, populations gradually increase in complexity and relative competency. At the individual level, organisms grow from a single-celled egg and exhibit extreme postnatal change as they interact with the outside world during their lifetimes. -

Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B
Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B. M ü ller, G ü nter P. Wagner, and Werner Callebaut, editors The Evolution of Cognition , edited by Cecilia Heyes and Ludwig Huber, 2000 Origination of Organismal Form: Beyond the Gene in Development and Evolutionary Biology , edited by Gerd B. M ü ller and Stuart A. Newman, 2003 Environment, Development, and Evolution: Toward a Synthesis , edited by Brian K. Hall, Roy D. Pearson, and Gerd B. M ü ller, 2004 Evolution of Communication Systems: A Comparative Approach , edited by D. Kimbrough Oller and Ulrike Griebel, 2004 Modularity: Understanding the Development and Evolution of Natural Complex Systems , edited by Werner Callebaut and Diego Rasskin-Gutman, 2005 Compositional Evolution: The Impact of Sex, Symbiosis, and Modularity on the Gradualist Framework of Evolution , by Richard A. Watson, 2006 Biological Emergences: Evolution by Natural Experiment , by Robert G. B. Reid, 2007 Modeling Biology: Structure, Behaviors, Evolution , edited by Manfred D. Laubichler and Gerd B. M ü ller, 2007 Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability in Human and Animal Communication , edited by Kimbrough D. Oller and Ulrike Griebel, 2008 Functions in Biological and Artifi cial Worlds: Comparative Philosophical Perspectives , edited by Ulrich Krohs and Peter Kroes, 2009 Cognitive Biology: Evolutionary and Developmental Perspectives on Mind, Brain, and Behavior , edited by Luca Tommasi, Mary A. Peterson, and Lynn Nadel, 2009 Innovation in Cultural Systems: Contributions from Evolutionary Anthropology , edited by Michael J. O ’ Brien and Stephen J. Shennan, 2010 The Major Transitions in Evolution Revisited , edited by Brett Calcott and Kim Sterelny, 2011 Transformations of Lamarckism: From Subtle Fluids to Molecular Biology , edited by Snait B. -

Study of Natural Longlife Juvenility and Tissue Regeneration in Caudate Amphibians and Potential Application of Resulting Data in Biomedicine
Journal of Developmental Biology Review Study of Natural Longlife Juvenility and Tissue Regeneration in Caudate Amphibians and Potential Application of Resulting Data in Biomedicine Eleonora N. Grigoryan Kol’tsov Institute of Developmental Biology, Russian Academy of Sciences, 119334 Moscow, Russia; [email protected]; Tel.: +7-(499)-1350052 Abstract: The review considers the molecular, cellular, organismal, and ontogenetic properties of Urodela that exhibit the highest regenerative abilities among tetrapods. The genome specifics and the expression of genes associated with cell plasticity are analyzed. The simplification of tissue structure is shown using the examples of the sensory retina and brain in mature Urodela. Cells of these and some other tissues are ready to initiate proliferation and manifest the plasticity of their phenotype as well as the correct integration into the pre-existing or de novo forming tissue structure. Without excluding other factors that determine regeneration, the pedomorphosis and juvenile properties, identified on different levels of Urodele amphibians, are assumed to be the main explanation for their high regenerative abilities. These properties, being fundamental for tissue regeneration, have been lost by amniotes. Experiments aimed at mammalian cell rejuvenation currently use various approaches. They include, in particular, methods that use secretomes from regenerating tissues of caudate amphibians and fish for inducing regenerative responses of cells. Such an approach, along with those developed on the basis of knowledge about the molecular and genetic nature and age dependence of regeneration, may become one more step in the development of regenerative medicine Citation: Grigoryan, E.N. Study of Keywords: salamanders; juvenile state; tissue regeneration; extracts; microvesicles; cell rejuvenation Natural Longlife Juvenility and Tissue Regeneration in Caudate Amphibians and Potential Application of Resulting Data in 1. -

Ecological Developmental Biology and Disease States CHAPTER 5 Teratogenesis: Environmental Assaults on Development 167
Integrating Epigenetics, Medicine, and Evolution Scott F. Gilbert David Epel Swarthmore College Hopkins Marine Station, Stanford University Sinauer Associates, Inc. • Publishers Sunderland, Massachusetts U.S.A. © Sinauer Associates, Inc. This material cannot be copied, reproduced, manufactured or disseminated in any form without express written permission from the publisher. Brief Contents PART 1 Environmental Signals and Normal Development CHAPTER 1 The Environment as a Normal Agent in Producing Phenotypes 3 CHAPTER 2 How Agents in the Environment Effect Molecular Changes in Development 37 CHAPTER 3 Developmental Symbiosis: Co-Development as a Strategy for Life 79 CHAPTER 4 Embryonic Defenses: Survival in a Hostile World 119 PART 2 Ecological Developmental Biology and Disease States CHAPTER 5 Teratogenesis: Environmental Assaults on Development 167 CHAPTER 6 Endocrine Disruptors 197 CHAPTER 7 The Epigenetic Origin of Adult Diseases 245 PART 3 Toward a Developmental Evolutionary Synthesis CHAPTER 8 The Modern Synthesis: Natural Selection of Allelic Variation 289 CHAPTER 9 Evolution through Developmental Regulatory Genes 323 CHAPTER 10 Environment, Development, and Evolution: Toward a New Synthesis 369 CODA Philosophical Concerns Raised by Ecological Developmental Biology 403 APPENDIX A Lysenko, Kammerer, and the Truncated Tradition of Ecological Developmental Biology 421 APPENDIX B The Molecular Mechanisms of Epigenetic Change 433 APPENDIX C Writing Development Out of the Modern Synthesis 441 APPENDIX D Epigenetic Inheritance Systems: -

Gabriel Dover)
Dear Mr Darwin (Gabriel Dover) Home | Intro | About | Feedback | Prev | Next | Search Steele: Lamarck's Was Signature Darwin Wrong? Molecular Drive: the Third Force in evolution Geneticist Gabriel Dover claims that there is a third force in evolution: 'Molecular Drive' beside natural selection and neutral drift. Molecular drive is operationally distinct from natural selection and neutral drift. According to Dover it explains biological phenomena, such as the 700 copies of a ribosomal RNA gene and the origin of the 173 legs of the centipede, which natural selection and neutral drift alone cannot explain. by Gert Korthof version 1.3 24 Mar 2001 Were Darwin and Mendel both wrong? Molecular Drive is, according to Dover, an important factor in evolution, because it shapes the genomes and forms of organisms. Therefore Neo-Darwinism is incomplete without Molecular Drive. It is no wonder that the spread of novel genes was ascribed to natural selection, because it was the only known process that could promote the spread of novel genes. Dover doesn't reject the existence of natural selection but points out cases where natural selection clearly fails as a mechanism. Molecular drive is a non-Darwinian mechanism because it is independent of selection. We certainly need forces in evolution, since natural selection itself is not a force. It is the passive outcome of other processes. It is not an active process, notwithstanding its name. Natural selection as an explanation is too powerful for its own good. Molecular drive is non-Mendelian because some DNA segments are multiplied disproportional. In Mendelian genetics genes are present in just two copies (one on the maternal and one on the paternal chromosome). -

Homeobox Gene Expression Profile in Human Hematopoietic Multipotent
Leukemia (2003) 17, 1157–1163 & 2003 Nature Publishing Group All rights reserved 0887-6924/03 $25.00 www.nature.com/leu Homeobox gene expression profile in human hematopoietic multipotent stem cells and T-cell progenitors: implications for human T-cell development T Taghon1, K Thys1, M De Smedt1, F Weerkamp2, FJT Staal2, J Plum1 and G Leclercq1 1Department of Clinical Chemistry, Microbiology and Immunology, Ghent University Hospital, Ghent, Belgium; and 2Department of Immunology, Erasmus Medical Center, Rotterdam, The Netherlands Class I homeobox (HOX) genes comprise a large family of implicated in this transformation proces.14 The HOX-C locus transcription factors that have been implicated in normal and has been primarily implicated in lymphomas.15 malignant hematopoiesis. However, data on their expression or function during T-cell development is limited. Using degener- Hematopoietic cells are derived from stem cells that reside in ated RT-PCR and Affymetrix microarray analysis, we analyzed fetal liver (FL) in the embryo and in the adult bone marrow the expression pattern of this gene family in human multipotent (ABM), which have the unique ability to self-renew and thereby stem cells from fetal liver (FL) and adult bone marrow (ABM), provide a life-long supply of blood cells. T lymphocytes are a and in T-cell progenitors from child thymus. We show that FL specific type of hematopoietic cells that play a major role in the and ABM stem cells are similar in terms of HOX gene immune system. They develop through a well-defined order of expression, but significant differences were observed between differentiation steps in the thymus.16 Several transcription these two cell types and child thymocytes. -

Evolving Neural Networks That Are Both Modular and Regular: Hyperneat Plus the Connection Cost Technique Joost Huizinga, Jean-Baptiste Mouret, Jeff Clune
Evolving Neural Networks That Are Both Modular and Regular: HyperNeat Plus the Connection Cost Technique Joost Huizinga, Jean-Baptiste Mouret, Jeff Clune To cite this version: Joost Huizinga, Jean-Baptiste Mouret, Jeff Clune. Evolving Neural Networks That Are Both Modular and Regular: HyperNeat Plus the Connection Cost Technique. GECCO ’14: Proceedings of the 2014 Annual Conference on Genetic and Evolutionary Computation, ACM, 2014, Vancouver, Canada. pp.697-704. hal-01300699 HAL Id: hal-01300699 https://hal.archives-ouvertes.fr/hal-01300699 Submitted on 11 Apr 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. To appear in: Proceedings of the Genetic and Evolutionary Computation Conference. 2014 Evolving Neural Networks That Are Both Modular and Regular: HyperNeat Plus the Connection Cost Technique Joost Huizinga Jean-Baptiste Mouret Jeff Clune Evolving AI Lab ISIR, Université Pierre et Evolving AI Lab Department of Computer Marie Curie-Paris 6 Department of Computer Science CNRS UMR 7222 Science University of Wyoming Paris, France University of Wyoming [email protected] [email protected] [email protected] ABSTRACT 1. INTRODUCTION One of humanity’s grand scientific challenges is to create ar- An open and ambitious question in the field of evolution- tificially intelligent robots that rival natural animals in intel- ary robotics is how to produce robots that possess the intel- ligence and agility. -

High Heritability of Telomere Length, but Low Evolvability, and No Significant
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.16.423128; this version posted December 16, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 High heritability of telomere length, but low evolvability, and no significant 2 heritability of telomere shortening in wild jackdaws 3 4 Christina Bauch1*, Jelle J. Boonekamp1,2, Peter Korsten3, Ellis Mulder1, Simon Verhulst1 5 6 Affiliations: 7 1 Groningen Institute for Evolutionary Life Sciences, University of Groningen, Nijenborgh 7, 8 9747AG Groningen, The Netherlands 9 2 present address: Institute of Biodiversity Animal Health & Comparative Medicine, College 10 of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, United 11 Kingdom 12 3 Department of Animal Behaviour, Bielefeld University, Morgenbreede 45, 33615 Bielefeld, 13 Germany 14 15 16 * Correspondence to: 17 [email protected] 18 19 Running head: High heritability of telomere length bioRxiv preprint doi: https://doi.org/10.1101/2020.12.16.423128; this version posted December 16, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 20 Abstract 21 Telomere length (TL) and shortening rate predict survival in many organisms. Evolutionary 22 dynamics of TL in response to survival selection depend on the presence of genetic variation 23 that selection can act upon. However, the amount of standing genetic variation is poorly known 24 for both TL and TL shortening rate, and has not been studied for both traits in combination in 25 a wild vertebrate. -
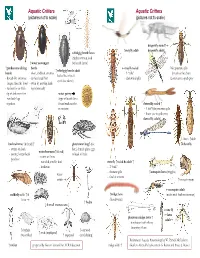
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1.