Vascular Pathology in the Renal Transplant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urinary System
OUTLINE 27.1 General Structure and Functions of the Urinary System 818 27.2 Kidneys 820 27 27.2a Gross and Sectional Anatomy of the Kidney 820 27.2b Blood Supply to the Kidney 821 27.2c Nephrons 824 27.2d How Tubular Fluid Becomes Urine 828 27.2e Juxtaglomerular Apparatus 828 Urinary 27.2f Innervation of the Kidney 828 27.3 Urinary Tract 829 27.3a Ureters 829 27.3b Urinary Bladder 830 System 27.3c Urethra 833 27.4 Aging and the Urinary System 834 27.5 Development of the Urinary System 835 27.5a Kidney and Ureter Development 835 27.5b Urinary Bladder and Urethra Development 835 MODULE 13: URINARY SYSTEM mck78097_ch27_817-841.indd 817 2/25/11 2:24 PM 818 Chapter Twenty-Seven Urinary System n the course of carrying out their specific functions, the cells Besides removing waste products from the bloodstream, the uri- I of all body systems produce waste products, and these waste nary system performs many other functions, including the following: products end up in the bloodstream. In this case, the bloodstream is ■ Storage of urine. Urine is produced continuously, but analogous to a river that supplies drinking water to a nearby town. it would be quite inconvenient if we were constantly The river water may become polluted with sediment, animal waste, excreting urine. The urinary bladder is an expandable, and motorboat fuel—but the town has a water treatment plant that muscular sac that can store as much as 1 liter of urine. removes these waste products and makes the water safe to drink. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -
![L8-Urine Conc. [PDF]](https://docslib.b-cdn.net/cover/4402/l8-urine-conc-pdf-1384402.webp)
L8-Urine Conc. [PDF]
The loop of Henle is referred to as countercurrent multiplier and vasa recta as countercurrent exchange systems in concentrating and diluting urine. Explain what happens to osmolarity of tubular fluid in the various segments of the loop of Henle when concentrated urine is being produced. Explain the factors that determine the ability of loop of Henle to make a concentrated medullary gradient. Differentiate between water diuresis and osmotic diuresis. Appreciate clinical correlates of diabetes mellitus and diabetes insipidus. Fluid intake The total body water Antidiuretic hormone is controled by : Renal excretion of water Hyperosmolar medullary Changes in the osmolarity of tubular fluid : interstitium 1 2 3 Low osmolarity The osmolarity High osmolarity because of active decrease as it goes up because of the transport of Na+ and because of the reabsorbation of water co-transport of K+ and reabsorption of NaCl Cl- 4 5 Low osmolarity because of High osmolarity because of reabsorption of NaCl , also reabsorption of water in reabsorption of water in present of ADH , present of ADH reabsorption of urea Mechanisms responsible for creation of hyperosmolar medulla: Active Co- Facilitated diffusion transport : transport : diffusion : of : Na+ ions out of the Only of small thick portion of the K+ , Cl- and other amounts of water ascending limb of ions out of the thick from the medullary the loop of henle portion of the Of urea from the tubules into the into the medullary ascending limb of inner medullary medullary interstitium the loop of henle collecting -

The Distal Convoluted Tubule and Collecting Duct
Chapter 23 *Lecture PowerPoint The Urinary System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Urinary system rids the body of waste products. • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filters blood plasma, separates waste from useful chemicals, returns useful substances to blood, eliminates wastes • Regulate blood volume and pressure by eliminating or conserving water • Regulate the osmolarity of the body fluids by controlling the relative amounts of water and solutes -

Introduction to the Urinary System
Tips for Success 1. Show up 2. Participate in lab 3. Show up 4. Turn in assignments (completed, refer to #6) 5. Show up 6. Communicate with me, e-mail is best 7. Show up Every point counts! Business Homework due in lab Label the handout provided today Front and back Use your book! There should be at least 18 items labeled Part 1 Urinary System Wastes Gases versus fluids Urinary system Dispose of water soluble wastes Electrolyte regulation Acid-base regulation Urinary System Other functions Kidneys Renin Erythropoietin Vitamin D activation Nitrogenous Wastes Urine is about 95% water Second largest component is urea Urea derived from breakdown of amino acids Nitrogenous Wastes TOXIC! + 1. Dietary amino acids → NH2 removed → NH2 + H → NH3 500 ml of urine removes only 1 gram of nitrogen as ammonia 2. Ammonia can be converted to urea Requires energy 50 ml of urine removes 1 gram of nitrogen as urea 3. Ammonia can be converted to uric acid Requires lots of energy 10 ml of urine removes 1 gram of nitrogen as uric acid Urinary System Organs Kidneys Major excretory organs Urinary bladder Temporary storage reservoir for urine Ureters Transport urine from the kidneys to the bladder Urethra Transports urine out of the body Hepatic veins (cut) Esophagus (cut) Inferior vena cava Renal artery Adrenal gland Renal hilum Aorta Renal vein Kidney Iliac crest Ureter Rectum (cut) Uterus (part of female reproductive Urinary system) bladder Urethra Figure 25.1 Kidney: Urinary System page 6 Anterior Inferior vena cava Peritoneal -

Lecture (1) Urinary System
UrinaryUrinaryUrinary systemsystemsystem Dr. Carmen E. Rexach Anatomy 35 Mt. San Antonio College Functions •Storage of urine – Bladder stores up to 1 L of urine • Excretion of urine – Transport of urine out of body • Blood volume regulation – Effects of hormones on kidneys • Regulation of erythrocyte production –Kidneys • Monitor oxygen content of blood • Produce EPO = erthrocyte production Components •Kidneys • Ureters • Urinary Bladder • Urethra Kidneys Gross Anatomy • Kidneys approx weight = 125- 150g each • Retroperitoneal – Anterior surface covered with peritoneum – Posterior surface directly against posterior abdominal wall • Superior surface at about T12 • Inferior surface at about L3 • ureters enter urinary bladder posteriorly • Left kidney 2cm superior to right –Size of liver Transverse section at L1 surface features of kidney • Hilum = the depression along the medial border through which several structures pass –renal artery –renal vein –ureter – renal nerves Surrounding structures • Fibrous capsule – Innermost layer of dense irregular CT – Maintains shape, protection • Adipose capsule (perinephric fat) – Adipose ct of varying thickness – Cushioning and insulation • Renal fascia – Dense irregular CT – Anchors kidney to peritoneum & abdominal wall • Paranephric fat – Outermost, adipose CT between renal fascia and peritoneum Coronal section •Cortex – layer of renal tissue in contact with capsule –Lighter shade –Renal columns= parts of cortex that extend into the medulla between pyramids •Medulla –Innermost – striped due to renal tubules •renal pyramids – 8-15 present in medulla of adult – conical shape – Wide base at corticomedullary junction Coronal section • Renal pelvis – collects from calyces, passes onto ureter •Calyces (pl) – funnel shaped regions – collect urine into pelvis •Minor calyx (s) – in contact with each pyramid •Major calyx (s) – collect from minor Microscopic Anatomy Microscopic anatomy Renal tubules • Nephron – functional unit of the kidney. -

Human Anatomy and Physiology II Laboratory
Human Anatomy and Physiology II Laboratory Anatomy of the Urinary System 1 This lab involves the exercise in the lab manual entitled “Anatomy of the Urinary System”. In this lab you will look at urinary system histology, and anatomy. Complete the review sheet from the exercise and take the online urinary system quiz. As an alternate your instructor may have you submit a drawing of kidney tissue from the Virtual Microsocpe or other histology site. There are also videos showing cadaver dissection of the urinary tract and sheep kidney. Click on the sound icon for the audio file (mp3 format) for each slide. There is also a link to a dowloadable mp4 video which can be played on an iPod. Organs of The Adrenal gland Urinary System Renal artery and vein Maintains homeostasis Kidney of the blood. Carries urine to the bladder Ureter Stores urine Urinary bladder Expels urine from the bladder Urethra 2 Structure of the Kidney Pyramid} Medulla Renal column Calyx Renal pelvis Papilla Cortex Ureter 3 The kidney is composed of several layers and is covered with a fibrous capsule, the renal capsule. The outer layer of the kidney is the cortex. It contains the major (upper) portion of the nephrons. The middle layer of the kidney is the medulla. It is composed of the triangular shaped pyramids and the renal columns. The pyramids contain the collecting tubules and loops of Henle, the lower portion of the nephrons. These tubules run nearly parallel to one another and give the pyramids a grain which leads to their points or papillae. -

Vascular Anatomy Defines Sites of Indomethacin Induced Jejunal Ulceration Along the Mesenteric Margin
Gut 1997; 41: 763–770 763 Vascular anatomy defines sites of indomethacin induced jejunal ulceration along the mesenteric Gut: first published as 10.1136/gut.41.6.763 on 1 December 1997. Downloaded from margin A Anthony, R E Pounder, A P Dhillon, A J Wakefield Abstract margin of the jejunum2–6 and more specifically, Background—Indomethacin induces ul- at sites supplied by short feeding vessels (vasa ceration in the rat jejunum with sparing of brevia, VB) located between the long feeding the ileum. The ulcers localise between vessels (vasa recta, VR).3 It is of interest that in vasa recta along the mesenteric margin of Crohn’s disease small intestinal ulcers also the bowel, observations that have not been tend to arise along the mesenteric margin fully explained. where blood vessels pass through the muscula- Aim—To examine the relationship be- ris propria, an acknowledged, but largely tween the localisation of experimental ignored observation.7 ulcers and the vascular anatomy of the rat Early anatomical studies of the human small intestine. gastrointestinal tract identified the existence of Methods—The normal vascular anatomy long (VR) and short (VB) feeding vessels that of the rat jejunum and ileum was studied supplied the antimesenteric and mesenteric and compared using arterial carbon ink margins of the bowel wall respectively.89While perfusion. The anatomical localisation of the mesenteric margin is known to be preferen- early and advanced lesions induced by tially ulcerated in the indomethacin/rat model indomethacin was examined with particu- this predilection still lacks an explanation. lar reference to the vasculature. Mucosal In this paper we have compared the normal injury induced by feeding vessel ligation vascular anatomy of the rat jejunum with the for 24 hours or brief ischaemia- ileum (sensitive and resistant sites of in- reperfusion injury was examined. -
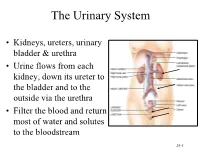
Urinary System
The Urinary System • Kidneys, ureters, urinary bladder & urethra • Urine flows from each kidney, down its ureter to the bladder and to the outside via the urethra • Filter the blood and return most of water and solutes to the bloodstream 26-1 Overview of Kidney Functions • Regulation of blood ionic composition – Na+, K+, Ca+2, Cl- and phosphate ions • Regulation of blood pH, osmolarity & glucose • Regulation of blood volume – conserving or eliminating water • Regulation of blood pressure • Release of erythropoietin & calcitriol • Excretion of wastes & foreign substances 26-2 Internal Anatomy of the Kidneys • Parenchyma of kidney – renal cortex = superficial layer of kidney – renal medulla • inner portion consisting of 8-18 cone-shaped renal pyramids separated by renal columns • renal papilla point toward center of kidney • Drainage system fills renal sinus cavity – cuplike structure (minor calyces) collect urine from the papillary ducts of the papilla – minor & major calyces empty into the renal pelvis which empties into the ureter 26-3 Internal Anatomy of Kidney Human Kidney 26-5 Blood & Nerve Supply of Kidney • Abundantly supplied with blood vessels – receive 25% of resting cardiac output via renal arteries • Functions of different capillary beds – glomerular capillaries where filtration of blood occurs – peritubular capillaries that carry away reabsorbed substances from filtrate (renal cortex) – vasa recta supplies nutrients to medulla • Sympathetic vasomotor nerves regulate blood flow by altering arterioles 26-6 Blood Vessels around -

The Urinary System Consists of Kidneys, Ureters, Urinary Bladder
Anatomy Lecture Notes Chapter 23 the urinary system consists of kidneys, ureters, urinary bladder, and urethra the function of the kidneys is NOT "to make urine" the kidneys: 1) regulate water balance 2) regular ECF electrolyte levels (Na, K, Ca) 3) eliminate some metabolic wastes urine is a by-product of these functions A. kidneys 1. located against posterior abdominal wall (retroperitoneal) T11 or T12 to L3 right kidney lower than left kidney 2. surrounded by a. pararenal fat (posterior only) b. renal fascia c. adipose capsule - perirenal fat d. renal capsule - dense c.t. covering surface of kidney e. parietal peritoneum Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 3. layers a. cortex - contains renal corpuscles and extends inwards as renal columns b. medulla - consists of renal pyramids which consist mostly of collecting ducts papilla - apex of renal pyramid; where collecting ducts drain into calyx 4. cavities and associated structures a. renal sinus - space in medial part of kidney; contains renal pelvis b. renal pelvis - expanded superior part of ureter minor calyx collects urine from one renal papilla major calyx formed by junction of 2 or more minor calyces renal pelvis formed by junction of all major calyces Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 5. renal hilum - medial indentation; where ureter leaves kidney 6. blood flow through the kidney - renal fraction = 20% of cardiac output aorta renal artery segmental arteries lobar arteries interlobar arteries arcuate arteries cortical radiate (interlobular) arteries afferent arterioles glomerular capillaries (glomerulus) efferent arteriole peritubular capillaries and vasa recta cortical radiate (interlobular) veins arcuate veins interlobar veins renal vein inferior vena cava Strong/Fall 2008 Anatomy Lecture Notes Chapter 23 7. -

Vascular Heterogeneity in the Kidney
Vascular Heterogeneity in the Kidney Grietje Molema, PhD,*,‡ and William C. Aird, MD*,§ Summary: Blood vessels and their endothelial lining are uniquely adapted to the needs of the underlying tissue. The structure and function of the vasculature varies both between and within different organs. In the kidney, the vascular architecture is designed to function both in oxygen/nutrient delivery and filtration of blood according to the homeostatic needs of the body. Here, we review spatial and temporal differences in renal vascular phenotypes in both health and disease. Semin Nephrol 32:145-155 © 2012 Published by Elsevier Inc. Keywords: Kidney, vasculature, endothelial cells, heterogeneity he blood vasculature has evolved to meet the kidney is configured not only to deliver oxygen and diverse needs of body tissues. As a result, the nutrients, but also to process blood for filtration. As a Tstructure and function of blood vessels and their result, renal blood flow is much greater than that which endothelial lining show remarkable heterogeneity both would be necessary to meet the metabolic demands of between and within different organs (reviewed by the organ: the kidneys comprise less than 1% of body Aird1,2). In most organs, blood vessels are organized in weight, but receive 25% of the cardiac output (re- prototypic series: arteries serve as conduits for bulk flow viewed by Evans et al3). Renal blood flow is five times delivery of blood; arterioles regulate resistance and thus that of basal coronary artery blood flow, yet renal blood flow; capillaries -

Aandp2ch23lecture.Pdf
Chapter 23 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Urinary system rids the body of waste products • Kidneys also play important roles in blood volume, pressure, and composition • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filter blood plasma, excrete toxic wastes • Regulate blood volume, pressure, and osmolarity • Regulate electrolytes and acid-base balance • Secrete erythropoietin, which stimulates the production of red blood cells • Help regulate calcium levels by participating in calcitriol synthesis • Clear hormones from blood • Detoxify free radicals • In starvation, they synthesize glucose from amino acids 23-5 Retroperitoneal Position of the Kidney Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display.