Plant Paleopolyploidy James C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
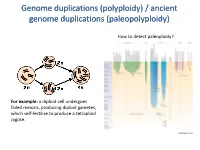
Polyploidy) / Ancient Genome Duplications (Paleopolyploidy
Genome duplications (polyploidy) / ancient genome duplications (paleopolyploidy) How to detect paleoploidy? For example: a diploid cell undergoes failed meiosis, producing diploid gametes, which self-fertilize to produce a tetraploid zygote. Timing of duplication by trees (phylogenetic timing) Phylogenetic timing of duplicates b Paramecium genome duplications Comparison of two scaffolds originating from a common ancestor at the recent WGD Saccharomyces cerevisiae Just before genome duplication Just after genome duplication More time after genome duplication Unaligned view (removing gaps just like in cerev has occurred) Saccharomyces cerevisiae Problem reciprocal gene loss (extreme case); how to solve? Problem reciprocal gene loss (extreme case); how to solve? Just before genome duplication Outgroup! Just after genome duplication Outgroup Just after genome duplication Outgroup More time after genome duplication Outgroup Problem (extreme case); how to solve? Outgroup Outgroup Outgroup Outgroup Outgroup Using other genomes Wong et al. 2002 PNAS Centromeres Vertebrate genome duplication Nature. 2011 Apr 10. [Epub ahead of print] Ancestral polyploidy in seed plants and angiosperms. Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, Depamphilis CW. Flowering plants Flowering MOSS Vertebrates Teleosts S. serevisiae and close relatives Paramecium Reconstructed map of genome duplications allows unprecedented mapping -

A 'David and Goliath'
Current Genomics, 2002, 3, 563-576 563 Nucleolar Dominance: A ‘David and Goliath’ Chromatin Imprinting Process W. Viegas1,*, N. Neves1,2, M. Silva1, A. Caperta1,2, and L. Morais-Cecílio1 1Centro de Botânica Aplicada à Agricultura, Secção de Genética/DBEB, Instituto Superior de Agronomia, 1349-017 Lisboa, 2Departamento de Ciências Biológicas e Naturais, Universidade Lusófona de Humanidades e Tecnologias, Campo Grande 376, 1749-042 Lisboa, Portugal Abstract: Nucleolar dominance is an enigma. The puzzle of differential amphiplasty has remained unresolved since it was first recognised and described in Crepis hybrids by Navashin in 1934. Here we review the body of knowledge that has grown out of the many models that have tried to find the genetic basis for differential rRNA gene expression in hybrids, and present a new interpretation. We propose and discuss a chromatin imprinting model which re-interprets differential amphiplasty in terms of two genomes of differing size occupying a common space within the nucleus, and with heterochromatin as a key player in the scenario. Difference in size between two parental genomes induces an inherited epigenetic mark in the hybrid that allows patterns of chromatin organization to have positional effects on the neighbouring domains. This chromatin imprinting model can be also used to explain complex genomic interactions which transcend nucleolar dominance and which can account for the overall characteristics of hybrids. Gene expression in hybrids, relative to parentage, is seen as being based on the nuclear location of the sequences concerned within their genomic environment, and where the presence of particular repetitive DNA sequences are ‘sensed’, and render silent the adjacent information. -

Gene Loss and Adaptation in Saccharomyces Genomes
Genetics: Published Articles Ahead of Print, published on December 1, 2005 as 10.1534/genetics.105.048900 After the duplication: gene loss and adaptation in Saccharomyces genomes Paul F. Cliften*,1, Robert S. Fulton§, Richard K. Wilson*, §, and Mark Johnston* *Department of Genetics and §Genome Sequencing Center, Washington University School of Medicine, 660 South Euclid Ave., St. Louis, MO, 63110; 1Current address: Department of Biology, Utah State University, 5305 Old Main Hill, Logan, UT, 84322 Running head: Saccharomyces genomic duplications Key words: genomic duplication, comparative sequence analysis, Saccharomyces phylogeny, yeast Corresponding author: Mark Johnston Department of Genetics Campus Box 8232 Washington University Medical School 4566 Scott Ave. St. Louis, MO 63110 TEL: 314-362-2735 FAX: 314-362-2157 [email protected] ABSTRACT The ancient duplication of the Saccharomyces cerevisiae genome and subsequent massive loss of duplicated genes is apparent when it is compared to the genomes of related species that diverged before the duplication event. To learn more about the evolutionary effects of the duplication event, we compared the S. cerevisiae genome to other Saccharomyces genomes. We demonstrate that the whole genome duplication occurred before S. castellii diverged from S. cerevisiae. In addition to more accurately dating the duplication event, this finding allowed us to study the effects of the duplication on two separate lineages. Analyses of the duplication regions of the genomes indicate that most of the duplicated genes (approximately 85%) were lost before the speciation. Only a small amount of paralogous gene loss (4-6%) occurred after speciation. On the other hand, S. castellii appears to have lost several hundred genes that were not retained as duplicated paralogs. -

Checklist of the Vascular Plants of Redwood National Park
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 9-17-2018 Checklist of the Vascular Plants of Redwood National Park James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Checklist of the Vascular Plants of Redwood National Park" (2018). Botanical Studies. 85. https://digitalcommons.humboldt.edu/botany_jps/85 This Flora of Northwest California-Checklists of Local Sites is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A CHECKLIST OF THE VASCULAR PLANTS OF THE REDWOOD NATIONAL & STATE PARKS James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State Univerity Arcata, California 14 September 2018 The Redwood National and State Parks are located in Del Norte and Humboldt counties in coastal northwestern California. The national park was F E R N S established in 1968. In 1994, a cooperative agreement with the California Department of Parks and Recreation added Del Norte Coast, Prairie Creek, Athyriaceae – Lady Fern Family and Jedediah Smith Redwoods state parks to form a single administrative Athyrium filix-femina var. cyclosporum • northwestern lady fern unit. Together they comprise about 133,000 acres (540 km2), including 37 miles of coast line. Almost half of the remaining old growth redwood forests Blechnaceae – Deer Fern Family are protected in these four parks. -
Plant List for Web Page
Stanford Working Plant List 1/15/08 Common name Botanical name Family origin big-leaf maple Acer macrophyllum Aceraceae native box elder Acer negundo var. californicum Aceraceae native common water plantain Alisma plantago-aquatica Alismataceae native upright burhead Echinodorus berteroi Alismataceae native prostrate amaranth Amaranthus blitoides Amaranthaceae native California amaranth Amaranthus californicus Amaranthaceae native Powell's amaranth Amaranthus powellii Amaranthaceae native western poison oak Toxicodendron diversilobum Anacardiaceae native wood angelica Angelica tomentosa Apiaceae native wild celery Apiastrum angustifolium Apiaceae native cutleaf water parsnip Berula erecta Apiaceae native bowlesia Bowlesia incana Apiaceae native rattlesnake weed Daucus pusillus Apiaceae native Jepson's eryngo Eryngium aristulatum var. aristulatum Apiaceae native coyote thistle Eryngium vaseyi Apiaceae native cow parsnip Heracleum lanatum Apiaceae native floating marsh pennywort Hydrocotyle ranunculoides Apiaceae native caraway-leaved lomatium Lomatium caruifolium var. caruifolium Apiaceae native woolly-fruited lomatium Lomatium dasycarpum dasycarpum Apiaceae native large-fruited lomatium Lomatium macrocarpum Apiaceae native common lomatium Lomatium utriculatum Apiaceae native Pacific oenanthe Oenanthe sarmentosa Apiaceae native 1 Stanford Working Plant List 1/15/08 wood sweet cicely Osmorhiza berteroi Apiaceae native mountain sweet cicely Osmorhiza chilensis Apiaceae native Gairdner's yampah (List 4) Perideridia gairdneri gairdneri Apiaceae -
Molecular Genetic Studies in Flax (Linum Usitatissimum L.)
Molecular genetic studies in flax (Linum usitatissimum L.) Moleculair genetische studies in vlas (Linum usitatissimum L.) Promotor: Prof. dr. ir. P. Stam Hoogleraar in de Plantenveredeling Copromotor: Dr. ir. H.J. van Eck Universitair docent, Laboratorium voor plantenveredeling Promotiecommissie: Prof. dr. A.G.M. Gerats (Radboud Universiteit Nijmegen) Prof. dr. R.F. Hoekstra (Wageningen Universiteit) Dr. R.G. van den Berg (Wageningen Universiteit) Dr. ir. J.W. van Ooijen (Kyazma B.V., Wageningen) Dit onderzoek is uitgevoerd binnen de onderzoeksscholen ‘Experimental Plant Sciences’ en ‘Production Ecology and Resource Conservation’. Molecular genetic studies in flax (Linum usitatissimum L.) Jaap Vromans Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. Dr. M.J. Kropff, in het openbaar te verdedigen op woensdag 15 maart 2006 des namiddags te vier uur in de Aula CIP-DTA Koninklijke Bibliotheek, Den Haag Molecular genetic studies in flax (Linum usitatissimum L.) Jaap Vromans PhD thesis, Wageningen University, The Netherlands With references – with summaries in Dutch and English ISBN 90-8504-374-3 Contents Chapter 1 General introduction 7 Chapter 2 Molecular analysis of flax (Linum usitatissimum L.) reveals a narrow genetic basis of fiber flax 21 Chapter 3 The molecular genetic variation in the genus Linum 41 Chapter 4 Selection of highly polymorphic AFLP fingerprints in flax (Linum usitatissimum L.) reduces the workload in genetic linkage map construction and results in -

Iv Gene Duplication and the Evolution of Silenced
Gene Duplication and the Evolution of Silenced Chromatin in Yeasts by Meleah A. Hickman University Program in Genetics and Genomics Duke University Date:_______________________ Approved: ___________________________ Laura Rusche, Advisor ___________________________ Paul Magwene, Chair ___________________________ Fred Dietrich ___________________________ Joe Heitman ___________________________ Beth Sullivan Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University Program in Genetics and Genomics in the Graduate School of Duke University 2010 i v ABSTRACT Gene Duplication and the Evolution of Silenced Chromatin in Yeasts by Meleah A. Hickman University Program in Genetics and Genomics Duke University Date:_______________________ Approved: ___________________________ Laura Rusche, Advisor ___________________________ Paul Magwene, Chair ___________________________ Fred Dietrich ___________________________ Joe Heitman ___________________________ Beth Sullivan An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University Program in Genetics and Genomics in the Graduate School of Duke University 2010 Copyright by Meleah A. Hickman 2010 Abstract In Saccharomyces cerevisiae, proper maintenance of haploid cell identity requires the SIR complex to mediate the silenced chromatin found at the cryptic mating-type loci, HML and HMR. This complex consists of Sir2, a histone deacetylase, and the histone binding proteins Sir3 and Sir4. Interestingly, both Sir2 and Sir3 have paralogs from a genome duplication that occurred after the divergence of Saccharomyces and Kluyveromyces species, approximately 100 million years ago. The histone deacetylase HST1 is the paralog of SIR2 and works with the promoter-specific SUM1 complex to repress sporulation and alpha-specific genes. ORC1 is the paralog of SIR3 and is an essential subunit of the Origin Recognition Complex and also recruits SIR proteins to the HM loci. -

Whole-Genome Duplications and Paleopolyploidy Whole-Genome Duplications
Whole-genome duplications and paleopolyploidy Whole-genome duplications AUTOPOLYPLOIDY ALLOPOLYPLOIDY Hegarty and HiscockHegarty 2008, 18 Current Biology Examples of allopolyploid speciation Hegarty and Hiscock 2008, Current Biology 18 Whole-genome duplications of different age time neopolyploidy mesopolyploidy paleopolyploidy time Whole-genome duplications in protozoa •Aury et al. (2006) analyzed the unicellular eukaryote Paramecium tetraurelia • most of 40,000 genes arose through at least 3 successive whole-genome duplications (WGDs) • most recent duplication most likely caused an explosion of speciation events that gave rise to the P. aurelia complex (15 sibling species) • some genes have been lost, some retained • many retained (duplicated) genes do not generate functional innovations but are important because of the gene dosage effect Whole-genome duplications in yeast • genome comparison between two yeast species, Saccharomyces cerevisiae (n=16) and Kluyveromyces waltii (n=8) • each region of K. waltii corresponding to two regions of S. cerevisiae •the S. cerevisiae genome underwent a WGD after the two yeast species diverged • in nearly every case (95%), accelerated evolution was confined to only one of the two paralogues (= one of the paralogues retained an ancestral function, the other was free to evolve more rapidly and acquired a derived function) Kellis et al. 2004, Nature 428 Whole-genome duplications in yeast Kellis et al. 2004, Nature 428 a) after divergence from K. waltii, the Saccharomyces lineage underwent a genome duplication event (2 copies of every gene and chromosome) b) duplicated genes were mutated and some lost c) two copies kept for only a small minority of duplicated genes d) the conserved order of duplicated genes (nos. -

Model-Based Integration of Genomics and Metabolomics Reveals SNP Functionality in Mycobacterium Tuberculosis
Model-based integration of genomics and metabolomics reveals SNP functionality in Mycobacterium tuberculosis Ove Øyåsa,b,1, Sonia Borrellc,d,1, Andrej Traunerc,d,1, Michael Zimmermanne, Julia Feldmannc,d, Thomas Liphardta,b, Sebastien Gagneuxc,d, Jörg Stellinga,b, Uwe Sauere, and Mattia Zampierie,2 aDepartment of Biosystems Science and Engineering, ETH Zurich, 4058 Basel, Switzerland; bSIB Swiss Institute of Bioinformatics, 1015 Lausanne, Switzerland; cDepartment of Medical Parasitoloy and Infection Biology, Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; dUniversity of Basel, 4058 Basel, Switzerland; and eInstitute of Molecular Systems Biology, ETH Zurich, 8093 Zurich, Switzerland Edited by Ralph R. Isberg, Tufts University School of Medicine, Boston, MA, and approved March 2, 2020 (received for review September 12, 2019) Human tuberculosis is caused by members of the Mycobacterium infection of macrophages (29–32). Beyond analyses of individual tuberculosis complex (MTBC) that vary in virulence and transmis- laboratory strains, however, no systematic characterization and sibility. While genome-wide association studies have uncovered comparative analysis of intrinsic metabolic differences across several mutations conferring drug resistance, much less is known human-adapted MTBC clinical strains has been performed. about the factors underlying other bacterial phenotypes. Variation If the metabolic and other phenotypic diversity between in the outcome of tuberculosis infection and diseases has been MTBC strains contributes to and modulates pathogenicity, an attributed primarily to patient and environmental factors, but obvious question is: Which elements of the limited genetic di- recent evidence indicates an additional role for the genetic diver- versity in the MTBC are responsible for phenotypic strain di- sity among MTBC clinical strains. -

Flora Mediterranea 26
FLORA MEDITERRANEA 26 Published under the auspices of OPTIMA by the Herbarium Mediterraneum Panormitanum Palermo – 2016 FLORA MEDITERRANEA Edited on behalf of the International Foundation pro Herbario Mediterraneo by Francesco M. Raimondo, Werner Greuter & Gianniantonio Domina Editorial board G. Domina (Palermo), F. Garbari (Pisa), W. Greuter (Berlin), S. L. Jury (Reading), G. Kamari (Patras), P. Mazzola (Palermo), S. Pignatti (Roma), F. M. Raimondo (Palermo), C. Salmeri (Palermo), B. Valdés (Sevilla), G. Venturella (Palermo). Advisory Committee P. V. Arrigoni (Firenze) P. Küpfer (Neuchatel) H. M. Burdet (Genève) J. Mathez (Montpellier) A. Carapezza (Palermo) G. Moggi (Firenze) C. D. K. Cook (Zurich) E. Nardi (Firenze) R. Courtecuisse (Lille) P. L. Nimis (Trieste) V. Demoulin (Liège) D. Phitos (Patras) F. Ehrendorfer (Wien) L. Poldini (Trieste) M. Erben (Munchen) R. M. Ros Espín (Murcia) G. Giaccone (Catania) A. Strid (Copenhagen) V. H. Heywood (Reading) B. Zimmer (Berlin) Editorial Office Editorial assistance: A. M. Mannino Editorial secretariat: V. Spadaro & P. Campisi Layout & Tecnical editing: E. Di Gristina & F. La Sorte Design: V. Magro & L. C. Raimondo Redazione di "Flora Mediterranea" Herbarium Mediterraneum Panormitanum, Università di Palermo Via Lincoln, 2 I-90133 Palermo, Italy [email protected] Printed by Luxograph s.r.l., Piazza Bartolomeo da Messina, 2/E - Palermo Registration at Tribunale di Palermo, no. 27 of 12 July 1991 ISSN: 1120-4052 printed, 2240-4538 online DOI: 10.7320/FlMedit26.001 Copyright © by International Foundation pro Herbario Mediterraneo, Palermo Contents V. Hugonnot & L. Chavoutier: A modern record of one of the rarest European mosses, Ptychomitrium incurvum (Ptychomitriaceae), in Eastern Pyrenees, France . 5 P. Chène, M. -

Vascular Plants of Humboldt Bay's Dunes and Wetlands Published by U.S
Vascular Plants of Humboldt Bay's Dunes and Wetlands Published by U.S. Fish and Wildlife Service G. Leppig and A. Pickart and California Department of Fish Game Release 4.0 June 2014* www.fws.gov/refuge/humboldt_bay/ Habitat- Habitat - Occurs on Species Status Occurs within Synonyms Common name specific broad Lanphere- Jepson Manual (2012) (see codes at end) refuge (see codes at end) (see codes at end) Ma-le'l Units UD PW EW Adoxaceae Sambucus racemosa L. red elderberry RF, CDF, FS X X N X X Aizoaceae Carpobrotus chilensis (Molina) sea fig DM X E X X N.E. Br. Carpobrotus edulis ( L.) N.E. Br. Iceplant DM X E, I X Alismataceae lanceleaf water Alisma lanceolatum With. FM X E plantain northern water Alisma triviale Pursh FM X N plantain Alliaceae three-cornered Allium triquetrum L. FS, FM, DM X X E leek Allium unifolium Kellogg one-leaf onion CDF X N X X Amaryllidaceae Amaryllis belladonna L. belladonna lily DS, AW X X E Narcissus pseudonarcissus L. daffodil AW, DS, SW X X E X Anacardiaceae Toxicodendron diversilobum Torrey poison oak CDF, RF X X N X X & A. Gray (E. Greene) Apiaceae Angelica lucida L. seacoast angelica BM X X N, C X X Anthriscus caucalis M. Bieb bur chevril DM X E Cicuta douglasii (DC.) J. Coulter & western water FM X N Rose hemlock Conium maculatum L. poison hemlock RF, AW X I X Daucus carota L. Queen Anne's lace AW, DM X X I X American wild Daucus pusillus Michaux DM, SW X X N X X carrot Foeniculum vulgare Miller sweet fennel AW, FM, SW X X I X Glehnia littoralis (A. -

6.047/6.878 Lecture 4: Comparative Genomics I: Genome Annotation Using Evolutionary Signatures
6.047/6.878 Lecture 4: Comparative Genomics I: Genome Annotation Using Evolutionary Signatures Mark Smith (Partially adapted from notes by: Angela Yen, Christopher Robde, Timo Somervuo and Saba Gul) 9/18/12 1 Contents 1 Introduction 4 1.1 Motivation and Challenge......................................4 1.2 Importance of many closely{related genomes...........................4 2 Conservation of genomic sequences5 2.1 Functional elements in Drosophila .................................5 2.2 Rates and patterns of selection...................................5 3 Excess Constraint 6 3.1 Causes of Excess Constraint.....................................7 3.2 Modeling Excess Constraint.....................................8 3.3 Excess Constraint in the Human Genome.............................9 3.4 Examples of Excess Constraint................................... 10 4 Diversity of evolutionary signatures: An Overview of Selection Patterns 10 4.1 Selective Pressures On Different Functional Elements....................... 11 5 Protein{Coding Signatures 12 5.1 Reading{Frame Conservation (RFC)................................ 13 5.2 Codon{Substitution Frequencies (CSFs).............................. 14 5.3 Classification of Drosophila Genome Sequences.......................... 16 5.4 Leaky Stop Codons.......................................... 17 6 microRNA (miRNA) genes 19 6.1 Computational Challenge...................................... 20 6.2 Unusual miRNA Genes........................................ 21 7 Regulatory Motifs 22 7.1 Computationally Detecting