Gas Chromatographic Analysis of Some Psychoactive Indole Bases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011)
EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011) 912 final COMMISSION STAFF WORKING PAPER on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances Accompanying the document REPORT FROM THE COMMISSION on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances {COM(2011) 430 final} EN EN TABLE OF CONTENTS 1. Introduction...................................................................................................................3 2. Methodology.................................................................................................................4 3. Key findings from the 2002 evaluation of the Joint Action on synthetic drugs ...........5 4. Overview of notifications, types of substances and trends at EU level 2005-2010......7 5. Other EU legislation relevant for the regulation of new psychoactive substances.....12 6. Functioning of the Council Decision on new psychoactive substances .....................16 7. Findings of the survey among Member States............................................................17 7.1. Assessment of the Council Decision ..........................................................................17 7.2. Stages in the functioning of the Council Decision .....................................................18 7.3. National responses to new psychoactive substances ..................................................20 -

A General Approach to the Screening and Confirmation of Tryptamines And
Available online at www.sciencedirect.com Talanta 74 (2008) 512–517 A general approach to the screening and confirmation of tryptamines and phenethylamines by mass spectral fragmentation Bo-Hong Chen a, Ju-Tsung Liu b, Wen-Xiong Chen a, Hung-Ming Chen a, Cheng-Huang Lin a,∗ a Department of Chemistry, National Taiwan Normal University, 88 Sec. 4, Tingchow Road, Taipei, Taiwan b Forensic Science Center, Military Police Command, Department of Defense, Taipei, Taiwan Received 6 March 2007; received in revised form 12 June 2007; accepted 13 June 2007 Available online 19 June 2007 Abstract Certain characteristic fragmentations of tryptamines (indoleethylamine) and phenethylamines are described. Based on the GC–EI/MS, LC–ESI/ MS and MALDI/TOFMS, the mass fragmentations of 13 standard compounds, including ␣-methyltryptamine (AMT), N,N-dimethyltryptamine (DMT), 5-methoxy-␣-methyltryptamine (5-MeO-AMT), N,N-diethyltryptamine (DET), N,N-dipropyltryptamine (DPT), N,N-dibutyltryptamine (DBT), N,N-diisopropyltryptamine (DIPT), 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), 5-methoxy-N,N-diisopropyltryptamine (5-MeO- DIPT), methamphetamine (MAMP), 3,4-methylenedioxyamphetamine (3,4-MDA), 3,4-methylenedioxymethamphetamine (3,4-MDMA) and 2- methylamino-1-(3,4-methylenedioxyphenyl)butane (MBDB), were compared. As a result, the parent ions of these analytes were hard to be obtained by GC/MS whereas the protonated molecular ions can be observed clearly by means of ESI/MS and MALDI/TOFMS. Furthermore, two major + + + + characteristic fragmentations, namely and ␣-cleavage ([M +H] → [3-vinylindole] ) and -cleavage ([M +H] → [CH2N RN1RN2]), are produced when the ESI and MALDI modes are used, respectively. In the case of ESI/MS, the fragment obtained from ␣-cleavage is the major process. -

LSD), Which Produces Illicit Market in the USA
DEPENDENCE LIABILITY OF "NON-NARCOTIC 9 DRUGS 81 INDOLES The prototype drug in this subgroup (Table XVI) potentials. Ibogaine (S 212) has appeared in the is compound S 219, lysergide (LSD), which produces illicit market in the USA. dependence of the hallucinogen (LSD) type (see above). A tremendous literature on LSD exists which documents fully the dangers of abuse, which REFERENCES is now widespread in the USA, Canada, the United 304. Sandoz Pharmaceuticals Bibliography on Psychoto- Kingdom, Australia and many western European mimetics (1943-1966). Reprinted by the US countries (for references see Table XVI). LSD must Department of Health, Education, & Welfare, be judged as a very dangerous substance which has National Institute of Mental Health, Washington, no established therapeutic use. D.C. 305. Cerletti, A. (1958) In: Heim, R. & Wasson, G. R., Substances S 200-S 203, S 206, S 208, S 213-S 218 ed., Les champignons hallucinogenes du Mexique, and S 220-S 222 are isomers or congeners of LSD. pp. 268-271, Museum national d'Histoire natu- A number of these are much less potent than LSD in relle, Paris (Etude pharmacologique de la hallucinogenic effect or are not hallucinogenic at all psilocybine) (compounds S 203, S 213, S 216, S 217, S 220 and 306. Cohen, S. (1965) The beyond within. The LSD S 222) and accordingly carry a lesser degree of risk story. Atheneum, New York than LSD. None of these weak hallucinogens has 307. Cohen, S. & Ditman, K. S. (1963) Arch. gen. been abused. Other compounds are all sufficiently Psychiat., 8, 475 (Prolonged adverse reactions to potent to make it likely that they would be abused if lysergic acid diethylamide) 308. -
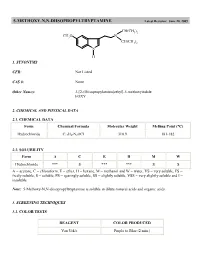
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

Hallucinogens: an Update
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Hallucinogens: An Update 146 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Hallucinogens: An Update Editors: Geraline C. Lin, Ph.D. National Institute on Drug Abuse Richard A. Glennon, Ph.D. Virginia Commonwealth University NIDA Research Monograph 146 1994 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGEMENT This monograph is based on the papers from a technical review on “Hallucinogens: An Update” held on July 13-14, 1992. The review meeting was sponsored by the National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the U.S. Department of Health and Human Services. The U.S. Government does not endorse or favor any specific commercial product or company. -

Update of the Generic Definition for Tryptamines
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Zahi Sulaiman 2nd Floor (NW), Seacole Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 1121 [email protected] Norman Baker MP, Minister for Crime Prevention Home Office 2 Marsham Street London SW1P 4DF 10 June 2014 Dear Minister, In December 2013, you commissioned the ACMD to begin a regular review of generic definitions under the Misuse of Drugs Act 1971, with the aim of capturing emerging new psychoactive substances. These drugs are variants of controlled drugs and fall outside the existing scope of the Misuse of Drugs Act 1971. The ACMD has considered evidence available on tryptamines in the context of the Misuse of Drugs Act 1971 and I enclose the Advisory Council’s advice and an expanded definition for tryptamine compounds with this letter. The ACMD’s NPS Committee has firstly reviewed previous research and existing controls to identify those tryptamines now seen to evade the existing controls. The ACMD has also reviewed data provided by the Home Office’s early warning systems and networks, clinical toxicology, prevalence and neuropharmacology in arriving at the expanded generic definition. This expanded generic definition will bring drugs such as alpha-methyltryptamine (AMT) as well as 5-MeO-DALT within the scope of the Misuse of Drugs Act 1971. These are highly potent hallucinogens which act on the 5HT2A receptor, in the same way as LSD. The ACMD therefore recommends that the tryptamines covered by the proposed expanded generic definition in this report, are controlled under the Misuse of Drugs Act (1971) as Class A substances. -

Test Purchase of New Synthetic Tryptamines Via the Internet: Identity Check by GC-MS and Separation by HPLC
Journal of Applied Pharmaceutical Science Vol. 6 (01), pp. 028-034, January, 2016 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2016.600105 ISSN 2231-3354 Test purchase of new synthetic tryptamines via the Internet: Identity check by GC-MS and separation by HPLC Magdalena Taschwer, Edith Ebner, Martin G Schmid* Department of Pharmaceutical Chemistry, Institute of Pharmaceutical Sciences, University of Graz, Universitätsplatz 1, A-8010 Graz, Austria. ABSTRACT ARTICLE INFO Article history: Over the past few years, a continuous alteration of the recreational drug market took place. Among other novel Received on: 25/09/2015 psychoactive drugs, new synthetic tryptamine derivatives appeared on the market. These compounds are mainly Revised on: 18/10/2015 traded via the Internet, which has become an important marketplace for the sale of recreational drugs. The goal of Accepted on: 08/11/2015 our research was to check, if 13 new synthetic tryptamines obtained by test purchase via different online vendors Available online: 26/01/2016 meet the promised identity. Analysis was performed by GC-MS, using a common 30 m HP-5MS capillary column as stationary phase. Subsequently, a simple HPLC method for the separation of these tryptamines was Key words: developed. Therefore, the aim was to establish a method to separate a broad spectrum of trypamines Tryptamines, Legal highs, simultaneously within short time. Measurements were performed by a LiChrospher® RP-18e column and a Novel psychoactive drugs, mobile phase consisting of 0.1% triethylammonium acetate buffer, methanol and acetonitrile. Both presented HPLC, GC-MS. methods were found to be suitable for the identification as well as separation of tryptamines as the analysis times were short and the selectivity sufficient. -

Alcohol and Drug Abuse Subchapter 9
Chapter 8 – Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority This rule is established under the authority of 18 V.S.A. §§ 4201 and 4202 which authorizes the Vermont Board of Health to designate regulated drugs for the protection of public health and safety. 2.0 Purpose This rule designates drugs and other chemical substances that are illegal or judged to be potentially fatal or harmful for human consumption unless prescribed and dispensed by a professional licensed to prescribe or dispense them and used in accordance with the prescription. The rule restricts the possession of certain drugs above a specified quantity. The rule also establishes benchmark unlawful dosages for certain drugs to provide a baseline for use by prosecutors to seek enhanced penalties for possession of higher quantities of the drug in accordance with multipliers found at 18 V.S.A. § 4234. 3.0 Definitions 3.1 “Analog” means one of a group of chemical components similar in structure but different with respect to elemental composition. It can differ in one or more atoms, functional groups or substructures, which are replaced with other atoms, groups or substructures. 3.2 “Benchmark Unlawful Dosage” means the quantity of a drug commonly consumed over a twenty-four-hour period for any therapeutic purpose, as established by the manufacturer of the drug. Benchmark Unlawful dosage is not a medical or pharmacologic concept with any implication for medical practice. Instead, it is a legal concept established only for the purpose of calculating penalties for improper sale, possession, or dispensing of drugs pursuant to 18 V.S.A. -
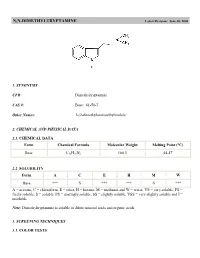
N,N-Dimethyltryptamine (DMT)
N,N-DIMETHYLTRYPTAMINE Latest Revision: June 30, 2000 1. SYNONYMS CFR: Dimethyltryptamine CAS #: Base: 61-50-7 Other Names: 3-(2-dimethylaminoethyl)indole 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Base C12H16N2 188.3 44-47 2.2. SOLUBILITY Form A C E H M W Base *** S *** *** S *** A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: Dimethyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Blue/violet 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative Rf COMPOUND System TLC 18 dimethyltryptamine 1.0 diethyltryptamine 1.9 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: 5% phenyl/95% methyl silicone 12 m x 0.2 mm x 0.33 µm film thickness Carrier gas: Helium at 1.0 mL/min Temperatures: Injector: 270°C Detector: 280°C Oven program: 1) 175°C initial temperature for 1.0 min 2) Ramp to 275°C at 15°C/min 3) Hold final temperature for 3.0 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in 4:1 chloroform: methanol and filtered. COMPOUND RRT COMPOUND RRT amphetamine 0.22 ketamine 1.18 methamphetamine 0.24 diphenhydramine 1.21 aspirin Breakdown 1 0.24 lidocaine 1.23 aspirin Breakdown 2 0.27 theophylline 1.41 nicotinamide 0.32 aspirin breakdown 5 1.43 ephedrine 0.37 chlorpheniramine 1.51 phenylpropanolamine 0.37 procaine 1.56 pseudoephedrine 0.37 cocaine 1.95 aspirin breakdown 3 0.44 triprolidine 2.07 3,4-MDMA 0.54 tetracosane 2.24 aspirin Breakdown 4 0.56 codeine 2.34 benzocaine 0.59 morphine 2.42 guaifenesin 0.74 hydrocodone 2.47 acetaminophen 0.76 hydromorphone 2.49 dimethyltryptamine 1.00 (2.62 min) oxycodone 2.62 caffeine 1.00 heroin 2.84 4. -

Maine State Legislature
MAINE STATE LEGISLATURE The following document is provided by the LAW AND LEGISLATIVE DIGITAL LIBRARY at the Maine State Law and Legislative Reference Library http://legislature.maine.gov/lawlib Reproduced from scanned originals with text recognition applied (searchable text may contain some errors and/or omissions) ONE HUNDRED AND FIFTH LEGISLATURE Legislative Document No. 790 H. P. 595 House of Representatives, February II, 1971 Referred to Committee on Judiciary. Sent up for concurrence and ordered printed. BERTHA W. JOHNSON, Clerk Presented by Mr. Rocheleau of Auburn. STATE OF MAINE IN THE YEAR OF OUR LORD NINETEEN HUNDRED SEVENTY-ONE AN ACT Relating to Possession of Certain Drugs. IBe it enacted by the People of the State of Maine, as follows: Sec. I. R. S., T. 22, § 2212-B, repealed and replaced. Section 2212-B of Title 22 of the Revised Statutes, as enacted by section 5 of chapter 390 of the public laws of 1967 and as amended by section 3 of chapter 443 of the public laws of 1969, is repealed and the following enacted in place thereof: § 2212-B. Possession of certain drugs Whoever, except the laboratory of the Department of Health and Welfare, and research centers and laboratories licensed under section 236S-A is found in possession of certain hallucinatory drugs shall upon conviction thereof be punished by a fine of not more than $1,000 or by imprisonment for not more than 2 years, or by both. Such hallucinatory drugs shall include any of the following substances, their optical isomers, and their salts: d-lysergic acid diethylamide -

Recreational Use, Analysis and Toxicity of Tryptamines
Send Orders for Reprints to [email protected] 26 Current Neuropharmacology, 2015, 13, 26-46 Recreational Use, Analysis and Toxicity of Tryptamines Roberta Tittarelli1, Giulio Mannocchi1, Flaminia Pantano1 and Francesco Saverio Romolo1,2,* 1Legal Medicine Section, Department of Anatomical, Histological, Forensic Medicine and Orthopedic Sciences, “Sapienza” University of Rome, Viale Regina Elena, 336, 00161 Rome, Italy; 2Institut de Police Scientifique, Université de Lausanne, Batochime, 1015 Lausanne, Switzerland Abstract: The definition New psychoactive substances (NPS) refers to emerging drugs whose chemical structures are similar to other psychoactive compounds but not identical, representing a “legal” alternative to internationally controlled drugs. There are many categories of NPS, such as synthetic cannabinoids, synthetic cathinones, phenylethylamines, piperazines, ketamine derivatives and tryptamines. Tryptamines are naturally occurring compounds, which can derive from the amino acid tryptophan by several biosynthetic pathways: their structure is a combination of a benzene ring Roberta Tittarelli and a pyrrole ring, with the addition of a 2-carbon side chain. Tryptamines include serotonin and melatonin as well as other compounds known for their hallucinogenic properties, such as psilocybin in ‘Magic mushrooms’ and dimethyltryptamine (DMT) in Ayahuasca brews. Aim: To review the scientific literature regarding tryptamines and their derivatives, providing a summary of all the available information about the structure of these compounds, their effects in relationship with the routes of administration, their pharmacology and toxicity, including articles reporting cases of death related to intake of these substances. Methods: A comprehensive review of the published scientific literature was performed, using also non peer-reviewed information sources, such as books, government publications and drug user web fora.