A Ten Years Follow-Up on Psychoactive Tryptamines Deliverance and Results of Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anaesthesia and Past Use Of
177 Correspondence were using LSD. It is more popular than other commonly Anaesthesia and past use of used hallucinogens whose quoted incidence of clients are: LSD ketamine 0.1% (super-K/vitamin K.I), psilocybin and psilocin 0.6% (the active alkaloids in the Mexican "magic To the Editor: mushroom"), and 3,4 methylenedioxymethamphetamine We report the case of a 43-yr-old lady who was admitted ~MDMA" 1% (ecstasy). The effects of the concurrent to the Day Surgery Unit for release of her carpal tunnel ingestion of LSD on anaesthesia are well described. 2-4 retinaculum. During the preoperative visit, she reported The long-term effects of the past use of LSD are largely no intercurrent illnesses, drug therapy or allergies. She unknown. We wonder if the hallucinations experienced did say, however, that she was frightened of general anaes- by our patient during anaesthesia were due to her LSD thesia, since she had experienced terrifying dreams during intake many years before. We would be interested to surgery under general anaesthesia on three occasions dur- know if others have had experience anaesthetising patients ing the previous ten years. On further questioning, she who are past users of phencyclidine-derived drugs. admitted that she had used lysergic acid diethylamide (LSD) during the late 1960's, the last occasion being 1968 Geoffrey N. Morris MRCGPFRCA when she had experienced characterstic hallucinations. Patrick T. Magee MSe FRCA She had not experienced hallucinations in the ensuing Anaesthetic Department years, except on the surgical occasions mentioned. Royal United Hospital One of the three previous operations had been per- Combe Park formed at our hospital and the anaesthetic record was Bath BA1 3NG checked. -

House Bill No. 1176
FIRST REGULAR SESSION HOUSE BILL NO. 1176 101ST GENERAL ASSEMBLY INTRODUCED BY REPRESENTATIVE DAVIS. 2248H.01I DANA RADEMAN MILLER, Chief Clerk AN ACT To repeal sections 191.480 and 579.015, RSMo, and to enact in lieu thereof two new sections relating to investigational drugs. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 191.480 and 579.015, RSMo, are repealed and two new sections 2 enacted in lieu thereof, to be known as sections 191.480 and 579.015, to read as follows: 191.480. 1. For purposes of this section, the following terms shall mean: 2 (1) "Eligible patient", a person who meets all of the following: 3 (a) Has a debilitating, life-threatening, or terminal illness; 4 (b) Has considered all other treatment options currently approved by the United States 5 Food and Drug Administration and all relevant clinical trials conducted in this state; 6 (c) Has received a prescription or recommendation from the person's physician for an 7 investigational drug, biological product, or device; 8 (d) Has given written informed consent which shall be at least as comprehensive as the 9 consent used in clinical trials for the use of the investigational drug, biological product, or device 10 or, if the patient is a minor or lacks the mental capacity to provide informed consent, a parent or 11 legal guardian has given written informed consent on the patient's behalf; and 12 (e) Has documentation from the person's physician that the person has met the 13 requirements of this subdivision; 14 (2) "Investigational drug, biological product, or device", a drug, biological product, or 15 device, any of which are used to treat the patient's debilitating, life-threatening, or terminal 16 illness, that has successfully completed phase one of a clinical trial but has not been approved EXPLANATION — Matter enclosed in bold-faced brackets [thus] in the above bill is not enacted and is intended to be omitted from the law. -

EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011)
EUROPEAN COMMISSION Brussels, 11.7.2011 SEC(2011) 912 final COMMISSION STAFF WORKING PAPER on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances Accompanying the document REPORT FROM THE COMMISSION on the assessment of the functioning of Council Decision 2005/387/JHA on the information exchange, risk assessment and control of new psychoactive substances {COM(2011) 430 final} EN EN TABLE OF CONTENTS 1. Introduction...................................................................................................................3 2. Methodology.................................................................................................................4 3. Key findings from the 2002 evaluation of the Joint Action on synthetic drugs ...........5 4. Overview of notifications, types of substances and trends at EU level 2005-2010......7 5. Other EU legislation relevant for the regulation of new psychoactive substances.....12 6. Functioning of the Council Decision on new psychoactive substances .....................16 7. Findings of the survey among Member States............................................................17 7.1. Assessment of the Council Decision ..........................................................................17 7.2. Stages in the functioning of the Council Decision .....................................................18 7.3. National responses to new psychoactive substances ..................................................20 -

Review of Psilocybin/Psilocin Pharmacology Isaac Dehart
Review of Psilocybin/Psilocin Pharmacology Isaac DeHart Introduction Psilocybin (PY, 4-phosphoryloxy-N,N-dimethyltryptamine or O-phosphoryl-4-hydroxy- N,N-dimethyltryptamine) (Figure 1, 1) is an indolamine (Figure 1, 2), and the primary ingredient in magic mushrooms. It has been implicated as a treatment for a number of psychological ailments including depression, general anxiety disorder, various addictions, obsessive- compulsive disorder, and post-traumatic stress disorder.1–4 Because of the drug’s re-emerging relevance in clinical applications, and because of its distinctive and informative effects on brain function, it is important to understand the pharmacology and general chemistry underlying its metabolism. Psilocybin is a prodrug of psilocin Magic mushrooms, the natural source of psilocybin may refer to several different hallucination-causing fungi, but the most potent of these are found in the genus Psilocybe. When ingested, magic mushrooms cause “trips,” wherein a user experiences euphoria, colorful hallucinations, and changes in perception, cognition and mood. In the case of “bad trips,” a user may experience states of intense panic or paranoia.5,6 More generally, these trips are sometimes described in terms of psychotic states, and behavioral studies involving psilocybin have demonstrated similarities between the effects of psilocybin and schizophrenia both in subjective experience and in physiological response.7 While psilocybin is more widely known as the cause behind these effects, it is referred to by researchers as a prodrug, -

An Old Chemical That Became a New Psychoactive Substance: Study on O-Acetylpsilocin Samples Handled for Analysis and Raise of Awareness
An old chemical that became a new psychoactive substance: study on O-Acetylpsilocin samples handled for analysis and raise of awareness A. Palma1,2, L.Galindo1, Marc Grifell1,2, P. Quintana3, A. Toll1,2, M. Ventura3, I. Fornís3, M. Torrens1,2,4, M.Farré4,5, F. Fonseca1,2 1 Institut de Neuropsiquiatria i Addiccions, Parc de Salut Mar, Barcelona, Spain. 2 Institut Hospital del Mar d'Investigacions Mèdiques, Parc de Salut Mar, Barcelona, Spain. 3 Asociación Bienestar y Desarrollo, Energy Control, Barcelona, Spain. 4 Universitat Autònoma de Barcelona, Barcelona, Spain. 5 Servei de Farmacología Clínica, Hospital Germans Trías i Pujol, Barcelona, Spain. Introduction Objective l New psychoactive substances (NPS) refer to emerging substances that have The aims of this study are appeared on the market and are not under international control (1). According to the m to explore the presence of 4-AcO-DMT from the samples handled to and data provided by the European Monitoring System for Drug and Drug Addiction, NPS analyzed by harm reduction service Energy Control and have experienced an unprecedented increase in number, type and availability during m to evaluate the ratio between 4-AcO-DMT and other related tryptamines the last years (2). (mushrooms, 4-AcO-DIPT, 4-AcO-MIPT and psilocybin). l Non-controlled tryptamines have psychedelic effects similar to the tryptamines already controlled such as psilocybin from Psylocybes mushrooms (3). l O-Acetylpsilocin also known as Psilacetin and 4-Acetoxy- Material and methods DMT (4-AcO-DMT) [Figure 1] is a synthetic tryptamine patented l Sample: all samples delivered for analysis from January 2009 to December in 1963 having a psychedelic effect by stimulating the 2014 were studied: serotoninergic system (3,4) and proposed as a research m handled as 4-AcO-DMT. -

A General Approach to the Screening and Confirmation of Tryptamines And
Available online at www.sciencedirect.com Talanta 74 (2008) 512–517 A general approach to the screening and confirmation of tryptamines and phenethylamines by mass spectral fragmentation Bo-Hong Chen a, Ju-Tsung Liu b, Wen-Xiong Chen a, Hung-Ming Chen a, Cheng-Huang Lin a,∗ a Department of Chemistry, National Taiwan Normal University, 88 Sec. 4, Tingchow Road, Taipei, Taiwan b Forensic Science Center, Military Police Command, Department of Defense, Taipei, Taiwan Received 6 March 2007; received in revised form 12 June 2007; accepted 13 June 2007 Available online 19 June 2007 Abstract Certain characteristic fragmentations of tryptamines (indoleethylamine) and phenethylamines are described. Based on the GC–EI/MS, LC–ESI/ MS and MALDI/TOFMS, the mass fragmentations of 13 standard compounds, including ␣-methyltryptamine (AMT), N,N-dimethyltryptamine (DMT), 5-methoxy-␣-methyltryptamine (5-MeO-AMT), N,N-diethyltryptamine (DET), N,N-dipropyltryptamine (DPT), N,N-dibutyltryptamine (DBT), N,N-diisopropyltryptamine (DIPT), 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), 5-methoxy-N,N-diisopropyltryptamine (5-MeO- DIPT), methamphetamine (MAMP), 3,4-methylenedioxyamphetamine (3,4-MDA), 3,4-methylenedioxymethamphetamine (3,4-MDMA) and 2- methylamino-1-(3,4-methylenedioxyphenyl)butane (MBDB), were compared. As a result, the parent ions of these analytes were hard to be obtained by GC/MS whereas the protonated molecular ions can be observed clearly by means of ESI/MS and MALDI/TOFMS. Furthermore, two major + + + + characteristic fragmentations, namely and ␣-cleavage ([M +H] → [3-vinylindole] ) and -cleavage ([M +H] → [CH2N RN1RN2]), are produced when the ESI and MALDI modes are used, respectively. In the case of ESI/MS, the fragment obtained from ␣-cleavage is the major process. -

LSD), Which Produces Illicit Market in the USA
DEPENDENCE LIABILITY OF "NON-NARCOTIC 9 DRUGS 81 INDOLES The prototype drug in this subgroup (Table XVI) potentials. Ibogaine (S 212) has appeared in the is compound S 219, lysergide (LSD), which produces illicit market in the USA. dependence of the hallucinogen (LSD) type (see above). A tremendous literature on LSD exists which documents fully the dangers of abuse, which REFERENCES is now widespread in the USA, Canada, the United 304. Sandoz Pharmaceuticals Bibliography on Psychoto- Kingdom, Australia and many western European mimetics (1943-1966). Reprinted by the US countries (for references see Table XVI). LSD must Department of Health, Education, & Welfare, be judged as a very dangerous substance which has National Institute of Mental Health, Washington, no established therapeutic use. D.C. 305. Cerletti, A. (1958) In: Heim, R. & Wasson, G. R., Substances S 200-S 203, S 206, S 208, S 213-S 218 ed., Les champignons hallucinogenes du Mexique, and S 220-S 222 are isomers or congeners of LSD. pp. 268-271, Museum national d'Histoire natu- A number of these are much less potent than LSD in relle, Paris (Etude pharmacologique de la hallucinogenic effect or are not hallucinogenic at all psilocybine) (compounds S 203, S 213, S 216, S 217, S 220 and 306. Cohen, S. (1965) The beyond within. The LSD S 222) and accordingly carry a lesser degree of risk story. Atheneum, New York than LSD. None of these weak hallucinogens has 307. Cohen, S. & Ditman, K. S. (1963) Arch. gen. been abused. Other compounds are all sufficiently Psychiat., 8, 475 (Prolonged adverse reactions to potent to make it likely that they would be abused if lysergic acid diethylamide) 308. -

Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com Ce4less.Com
Hallucinogens And Dissociative Drug Use And Addiction Introduction Hallucinogens are a diverse group of drugs that cause alterations in perception, thought, or mood. This heterogeneous group has compounds with different chemical structures, different mechanisms of action, and different adverse effects. Despite their description, most hallucinogens do not consistently cause hallucinations. The drugs are more likely to cause changes in mood or in thought than actual hallucinations. Hallucinogenic substances that form naturally have been used worldwide for millennia to induce altered states for religious or spiritual purposes. While these practices still exist, the more common use of hallucinogens today involves the recreational use of synthetic hallucinogens. Hallucinogen And Dissociative Drug Toxicity Hallucinogens comprise a collection of compounds that are used to induce hallucinations or alterations of consciousness. Hallucinogens are drugs that cause alteration of visual, auditory, or tactile perceptions; they are also referred to as a class of drugs that cause alteration of thought and emotion. Hallucinogens disrupt a person’s ability to think and communicate effectively. Hallucinations are defined as false sensations that have no basis in reality: The sensory experience is not actually there. The term “hallucinogen” is slightly misleading because hallucinogens do not consistently cause hallucinations. 1 ce4less.com ce4less.com ce4less.com ce4less.com ce4less.com ce4less.com ce4less.com How hallucinogens cause alterations in a person’s sensory experience is not entirely understood. Hallucinogens work, at least in part, by disrupting communication between neurotransmitter systems throughout the body including those that regulate sleep, hunger, sexual behavior and muscle control. Patients under the influence of hallucinogens may show a wide range of unusual and often sudden, volatile behaviors with the potential to rapidly fluctuate from a relaxed, euphoric state to one of extreme agitation and aggression. -
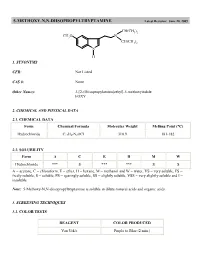
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005
5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE Latest Revision: June 20, 2005 CH(CH3)2 CH3O N CH(CH3)2 N H 1. SYNONYMS CFR: Not Listed CAS #: None Other Names: 3-[2-(Diisopropylamino)ethyl]-5-methoxyindole FOXY 2. CHEMICAL AND PHYSICAL DATA 2.1. CHEMICAL DATA Form Chemical Formula Molecular Weight Melting Point (°C) Hydrochloride C17H27N2OCl 310.9 181-182 2.2. SOLUBILITY Form A C E H M W Hydrochloride *** S *** *** S S A = acetone, C = chloroform, E = ether, H = hexane, M = methanol and W = water, VS = very soluble, FS = freely soluble, S = soluble, PS = sparingly soluble, SS = slightly soluble, VSS = very slightly soluble and I = insoluble Note: 5-Methoxy-N,N-diisopropyltryptamine is soluble in dilute mineral acids and organic acids. 3. SCREENING TECHNIQUES 3.1. COLOR TESTS REAGENT COLOR PRODUCED Van Urk's Purple to Blue (2 min.) 3.2. THIN LAYER CHROMATOGRAPHY Visualization Van Urk's reagent Relative R COMPOUND f System TLC 18 dimethyltryptamine 0.32 diethyltryptamine 0.89 5-methoxy- -methyltryptamine 0.70 5-methoxydiisopropyltryptamine 1.0 (8.05 cm) 3.3. GAS CHROMATOGRAPHY Method DMT-GCS1 Instrument: Gas chromatograph operated in split mode with FID Column: J&W DB-1 15 m x 0.32 mm x 0.25 µm film thickness Carrier gas: Helium at 1.3 mL/min Temperatures: Injector: 275°C Detector: 280°C Oven program: 190°C for 10 min Injection Parameters: Split Ratio = 60:1, 1 µL injected Samples are to be dissolved in chloroform, washed with dilute sodium carbonate and filtered. COMPOUND RRT COMPOUND RRT indole 0.12 DET 0.37 MDA 0.15 C-4 phthalate 0.38 MDMA 0.17 5-MeO-AMT 0.42 tryptamine 0.23 5-MeODMT 0.46 AMT 0.24 DIPT 0.58 DMT 0.26 C-5 phthalate 0.65 caffeine 0.28 5-MeODIPT 1.00 (7.14 min) 4. -

Hallucinogens: an Update
National Institute on Drug Abuse RESEARCH MONOGRAPH SERIES Hallucinogens: An Update 146 U.S. Department of Health and Human Services • Public Health Service • National Institutes of Health Hallucinogens: An Update Editors: Geraline C. Lin, Ph.D. National Institute on Drug Abuse Richard A. Glennon, Ph.D. Virginia Commonwealth University NIDA Research Monograph 146 1994 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health National Institute on Drug Abuse 5600 Fishers Lane Rockville, MD 20857 ACKNOWLEDGEMENT This monograph is based on the papers from a technical review on “Hallucinogens: An Update” held on July 13-14, 1992. The review meeting was sponsored by the National Institute on Drug Abuse. COPYRIGHT STATUS The National Institute on Drug Abuse has obtained permission from the copyright holders to reproduce certain previously published material as noted in the text. Further reproduction of this copyrighted material is permitted only as part of a reprinting of the entire publication or chapter. For any other use, the copyright holder’s permission is required. All other material in this volume except quoted passages from copyrighted sources is in the public domain and may be used or reproduced without permission from the Institute or the authors. Citation of the source is appreciated. Opinions expressed in this volume are those of the authors and do not necessarily reflect the opinions or official policy of the National Institute on Drug Abuse or any other part of the U.S. Department of Health and Human Services. The U.S. Government does not endorse or favor any specific commercial product or company. -

Update of the Generic Definition for Tryptamines
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen Secretary: Zahi Sulaiman 2nd Floor (NW), Seacole Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 1121 [email protected] Norman Baker MP, Minister for Crime Prevention Home Office 2 Marsham Street London SW1P 4DF 10 June 2014 Dear Minister, In December 2013, you commissioned the ACMD to begin a regular review of generic definitions under the Misuse of Drugs Act 1971, with the aim of capturing emerging new psychoactive substances. These drugs are variants of controlled drugs and fall outside the existing scope of the Misuse of Drugs Act 1971. The ACMD has considered evidence available on tryptamines in the context of the Misuse of Drugs Act 1971 and I enclose the Advisory Council’s advice and an expanded definition for tryptamine compounds with this letter. The ACMD’s NPS Committee has firstly reviewed previous research and existing controls to identify those tryptamines now seen to evade the existing controls. The ACMD has also reviewed data provided by the Home Office’s early warning systems and networks, clinical toxicology, prevalence and neuropharmacology in arriving at the expanded generic definition. This expanded generic definition will bring drugs such as alpha-methyltryptamine (AMT) as well as 5-MeO-DALT within the scope of the Misuse of Drugs Act 1971. These are highly potent hallucinogens which act on the 5HT2A receptor, in the same way as LSD. The ACMD therefore recommends that the tryptamines covered by the proposed expanded generic definition in this report, are controlled under the Misuse of Drugs Act (1971) as Class A substances. -

Test Purchase of New Synthetic Tryptamines Via the Internet: Identity Check by GC-MS and Separation by HPLC
Journal of Applied Pharmaceutical Science Vol. 6 (01), pp. 028-034, January, 2016 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2016.600105 ISSN 2231-3354 Test purchase of new synthetic tryptamines via the Internet: Identity check by GC-MS and separation by HPLC Magdalena Taschwer, Edith Ebner, Martin G Schmid* Department of Pharmaceutical Chemistry, Institute of Pharmaceutical Sciences, University of Graz, Universitätsplatz 1, A-8010 Graz, Austria. ABSTRACT ARTICLE INFO Article history: Over the past few years, a continuous alteration of the recreational drug market took place. Among other novel Received on: 25/09/2015 psychoactive drugs, new synthetic tryptamine derivatives appeared on the market. These compounds are mainly Revised on: 18/10/2015 traded via the Internet, which has become an important marketplace for the sale of recreational drugs. The goal of Accepted on: 08/11/2015 our research was to check, if 13 new synthetic tryptamines obtained by test purchase via different online vendors Available online: 26/01/2016 meet the promised identity. Analysis was performed by GC-MS, using a common 30 m HP-5MS capillary column as stationary phase. Subsequently, a simple HPLC method for the separation of these tryptamines was Key words: developed. Therefore, the aim was to establish a method to separate a broad spectrum of trypamines Tryptamines, Legal highs, simultaneously within short time. Measurements were performed by a LiChrospher® RP-18e column and a Novel psychoactive drugs, mobile phase consisting of 0.1% triethylammonium acetate buffer, methanol and acetonitrile. Both presented HPLC, GC-MS. methods were found to be suitable for the identification as well as separation of tryptamines as the analysis times were short and the selectivity sufficient.