Where Does All That Metal Come From?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oil Analysis Handbook Third Edition Copyright © 2017 Spectro Scientific
Predictive Equipment Maintenance Oil Analysis Handbook Third Edition Copyright © 2017 Spectro Scientific. All rights reserved. FluidScan® and LaserNet Fines® are registered trademarks of Spectro Scientific Inc. While every effort is made to assure the information in this document ois accurate, Spectro Scientific does not accept liability for any errors or mistakes that may arise. Specifications are subject to change without notice. | 3 Preface Welcome to the third edition of the In-service Oil Analysis Handbook. It has been a few years since the publication of the first edition of Spectro Scientific’s In-Service Oil Analysis Handbook. Our original goal was to compile a comprehensive reference book of common in-service oil analysis techniques to help readers understand and choose the right technique and instrumentation for their needs. We had to limit the scope in the first two editions because of the amount of effort needed to cover all the topics. In-service oil analysis for condition based maintenance covers a wide array of topics. I am pleased to say that in this third edition, we are much closer to our goal. We reorganized the structure of the content for better clarity and we added articles to cover more topics and instruments associated with oil analysis. Also, we rewrote several articles including the latest developments on the market. As we learned more from our customers about their successes using on site oil analysis, we developed case studies that you can find in this edition. This work is not possible without the time and effort from the contributing authors: Patrick Henning, Daniel Walsh, Robert Yurko, Ken Caldwell, Thomas Barraclough, Maria Bartus, Randi Price, John Morgan, Aifeng Shi and Yuegang Zhao from Spectro Scientific and Ray Garvey from Emerson Process Management. -

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

Synthetic Lubricant Base Stocks Formulations Guide Table of Contents
Synthetic lubricant base stocks formulations guide Table of contents 1.0 Introduction — Using this guide ����������������������������������������������������������������� 1 7�3 Turbine oils ��������������������������������������������������������������������������������������������62 2.0 Lubricant formulators FAQs ������������������������������������������������������������������������ 3 7�4 Industrial gear oils ��������������������������������������������������������������������������������67 7�5 Paper machine oils �������������������������������������������������������������������������������72 3.0 Synthetic base stock grade slate summary ���������������������������������������������� 7 7�6 Lubricants for use with food machinery 4.0 Industry trends ����������������������������������������������������������������������������������������������� 9 (incidental food contact) ������������������������������������������������������������������������������������74 4�1 Energy outlook ��������������������������������������������������������������������������������������� 9 7�7 Miscellaneous lubricants ���������������������������������������������������������������������79 4�2 Lubricant industry trends ��������������������������������������������������������������������10 7�7�1 Heat transfer oils �����������������������������������������������������������������������79 4�3 Automotive trends �������������������������������������������������������������������������������10 7�7�2 Chain lubricants �������������������������������������������������������������������������81 -

Polymerization of Safflower Oil in a Diesel Lubrication System by John Walter Olson a Thesis Submitted in Partial Fulfillment Of
Polymerization of safflower oil in a diesel lubrication system by John Walter Olson A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by John Walter Olson (1988) Abstract: Oxidative addition polymerization of lubrication oil contaminated with safflower oil diesel fuel was investigated in laboratory apparatus simulating conditions prevailing in a diesel engine crankcase. Primary research objectives were the identification and testing of key additives which might suppress problems of excessive viscosity rise and alkalinity decline of lubrication/safflower oil mixtures. Both commercial and base stock medium-speed locomotive lubrication oils were investigated with 5.0 weight percent safflower oil contamination. Oil mixtures were exposed to elevated temperature and were contacted by percolation with either oxygen or nitrogen to provide an oxidizing atmosphere and agitation or simply agitation. Copper, a known diesel engine wear metal, was introduced as a polymerization catalyst. Lubrication oils were evaluated with and without safflower oil and additives. Both commercial and base stock oils with no safflower oil were found to polymerize at a moderate baseline level. Amines were found to show little alkalinity maintenance efficacy while performance by an overbased calcium phenate was relatively good. Numerous antioxidants were tested for ability to disrupt the mechanisms leading to polymerization. Two widely-used antioxidants, zinc dialkyldithiophosphate and zinc diamyl-dithiocarbamate, were found to promote polymerization of oil mixtures in this research. This unexpected result is almost certainly a result of promotion catalysis by zinc. A metal deactivator N-N'-disalicylidene-1,2-propanediamine retarded viscosity rise, apparently by chelating wear metal catalysts. -

Chromium Compounds Hazard Summary
Chromium Compounds Hazard Summary Chromium occurs in the environment primarily in two valence states, trivalent chromium (Cr III) and hexavalent chromium (Cr VI). Exposure may occur from natural or industrial sources of chromium. Chromium III is much less toxic than chromium (VI). The respiratory tract is also the major target organ for chromium (III) toxicity, similar to chromium (VI). Chromium (III) is an essential element in humans. The body can detoxify some amount of chromium (VI) to chromium (III). The respiratory tract is the major target organ for chromium (VI) toxicity, for acute (short-term) and chronic (long-term) inhalation exposures. Shortness of breath, coughing, and wheezing were reported from a case of acute exposure to chromium (VI), while perforations and ulcerations of the septum, bronchitis, decreased pulmonary function, pneumonia, and other respiratory effects have been noted from chronic exposure. Human studies have clearly established that inhaled chromium (VI) is a human carcinogen, resulting in an increased risk of lung cancer. Animal studies have shown chromium (VI) to cause lung tumors via inhalation exposure. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (7), which contains information on inhalation chronic toxicity and the RfC and oral chronic toxicity and the RfD, and the carcinogenic effects of chromium including the unit cancer risk for inhalation exposure, EPA's Toxicological Review of Trivalent Chromium and Toxicological Review of Hexavalent Chromium (3), and the Agency for Toxic Substances and Disease Registry's (ATSDR's) Toxicological Profile for Chromium. (1) Uses The metal chromium is used mainly for making steel and other alloys. -

Mabanol Engine Oil Additive Motor Oil Additive
Mabanol Engine Oil Additive Motor Oil Additive Application Properties Mabanol Engine Oil Additive is filled into the Mabanol Engine Oil Additive is a nano tech- engine along with the motor oil. It is miscible nology-based oil additive designed to reduce with all types of motor oil. friction on mechanical parts, optimize engine It can be used in all petrol and diesel engines, performance and protect it in a durable way. including those equipped with catalytic con- Mabanol Engine Oil Additive produces a ro- verters, turbochargers and/or particulate filters bust lubricating film on the friction surfaces in both in passenger cars as well as in trucks. the engine which reduces friction losses and Not suitable for use in ATFs and motorcycles wear. This extends the life of the engine, im- with wet clutch. proves smoothness and optimizes efficiency. Mabanol Engine Oil Additive is also recom- mended for improving the performance of oil EAN Code in pumps, gearboxes and compressors. 300 ml can: 4260214890989 A can of 300 ml can be used for up to 4.5 liters of engine oil. The maximum dosage in the engine oil/additive blend is 20 volume percent. Please ensure that the correct oil level is maintained. Please shake well before use. Data Test method Unit Value Density at 20°C DIN 51 757 g/cm³ 0,874 Kin. Viscosity at 40°C DIN EN ISO 3104 mm²/s 316 Flash point ISO 3679 °C > 100 Lower explosion limit – Vol-% 0,6 Upper explosion limit – Vol-% 6,5 Updated in March 2019 The above values may vary within the commercial limits. -

(Copcs) Recommendations Fuel Additives Red Hill Bulk Fuel Storage Fuel Facility
Chemicals of Potential Concern (COPCs) Recommendations Fuel Additives Red Hill Bulk Fuel Storage Fuel Facility A meeting was held on May 10, 2016 to discuss the recommended approach to addressing the objectives of the Administrative Order on Consent (AOC) In the Matter of Red Hill Bulk Fuel Storage Facility (herein referred to as “the Facility”) Statement of Work (SOW) Section 6 and Section 7 with the Regulatory Agencies (State of Hawaii Department of Health [DOH] and United States Environmental Protection Agency Region IX [EPA]) and various subject matter experts (SMEs). The following attended the meeting: Parties of the AOC (Regulatory Agencies, Department of Navy [Navy], and Defense Logistics Agency [DLA]) and SMEs to the Regulatory Agencies (University of Hawaii [UH]; State of Hawaii Department of Land and Natural Resources [DLNR] Commission on Water Resources Management [COWRM]; United States Geological Survey [USGS] Pacific Islands Water Science Center; City and County of Honolulu Board of Water Supply [BWS]). Also in attendance were the Navy’s contractor, AECOM, and BWS’ contractor, Intera Geoscience & Engineering Solutions (Intera). One of the action items from the meeting was for the Navy and DLA to evaluate fuel additives and determine if additional analytes need to be included on the chemicals of potential concern (COPCs) list for the Facility, as previously agreed upon by the Parties of the AOC on February 4, 2016. The following discussion and table present the results of the fuel additives evaluation: Table 1 summarizes 18 chemical constituents of additives associated with fuel stored at the Facility. Six groups of fuel additives were identified and evaluated: (1) metal deactivators; (2) corrosion inhibitors and lubricity improvers; (3) icing inhibitors; (4) static dissipaters; (5) lubricity improvers; and (6) antioxidants. -

The Role of Nanoparticles in Lubricants; Performing Lubricated and Dry Friction Tests
FELLOWSHIP RESEARCH The Role of Nanoparticles in Lubricants; Performing Lubricated and Dry Friction Tests Hamed Ghaednia* and Dr. Robert L. Jackson (Advisor) Auburn University, Department of Mechanical Engineering, Auburn, Ala. *Ford Motor Co., Powertrain Research and Advanced Engineering Group, Dearborn, Mich. Editor’s Note: This month TLT profiles the 2014 recipient of The E. Elmer Klaus Fellowship, Hamed Ghaednia (Auburn University). The Klaus Fellowship, along with The E. Richard Booser Scholarship, are awarded annually to graduate and undergraduate students, respectively, who have an interest in pursuing a career in tribology. As a requirement for receiving an STLE scholarship, students are given the opportunity to participate in a tribology research project and to submit a report summarizing their research. Hamed Ghaednia graduated with a bachelor’s of science degree in mechanical and chemical INTRODUCTION engineering and a master’s of science degree Nanoparticles when suspended in in mechanical engineering from the Tehran a lubricant can infiltrate small gaps Polytechnic. He recently received his between rough surfaces in contact doctorate in mechanical engineering, with and alter the contact’s tribological an emphasis in tribology, from Auburn performance. Hence, nanoparticles University’s Samuel Ginn College of offer an alternative approach to Engineering’s tribology and lubrication lubrication by introducing third body science minor program. He is currently entities directly into the contact. The working as a tribology research engineer ability of nano-sized particles to pass at the Ford Motor Co. in the Powertrain through conventional filters, penetrate Research and Advanced Engineering Group. into contacts that larger particles You can reach him at [email protected]. -
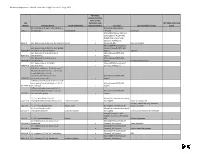
Chemicals of High Concern List (Sorted Alphabetically), July 2010
Minnesota Department of Health, Chemicals of High Concern list, July 1, 2010 Persistent, Bioaccumulative, Toxic or very CAS Persistent, very HPV (2006 and 3 of 4 Number Chemical Name Health endpoint(s) Bioaccumulative Source(s) Use example(s) or class years) (S)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2- Maine (EU Reproductive 5543-57-7 benzopyrone Reproduction Toxicant) Sunscreen Maine (CA Prop 65; IARC; EU Carcinogen; NTP 11th ROC; OSPAR Chemicals of High Concern); WA Appen1; 91-94-1 [1,1'-biphenyl]-4,4'-diamine, 3,3'-dichloro-Cancer x Minnesota HRL Dye, curing agent Maine (OSPAR Chemicals of [1,1'-biphenyl]-4,4'-diamine, N,N'-bis(2,4- Concern; Canada PBiT); WA 29398-96-7 dinitrophenyl)-3,3'-dimethoxy- x Appen1 Colorant [1,1'-Biphenyl]-4-ol, 3,4',5-tris(1,1- Maine (Canada PBiT); WA 6257-39-2 dimethylethyl)- x Appen1 [1,1'-Biphenyl]-4-ol, 3,4'-bis(1,1- Maine (Canada PBiT); WA 42479-88-9 dimethylethyl)- x Appen1 Chemical intermediate [1,1'-biphenyl]-4-ol, 3,5-bis(1,1- Maine (OSPAR Chemicals of 2668-47-5 dimethylethyl)- x Concern); WA Appen1 [2,6'-Bibenzothiazole]-7-sulfonic acid, 2'- (4-aminophenyl)-6-methyl-, diazotized, coupled with diazotized 4- aminobenzenesulfonic acid and Maine (Canada PBiT); WA 91696-90-1 resorcinol, sodium salts x Appen1 1(2H)-Quinolineethanol, 6-[(2-chloro-4,6- dinitrophenyl) azo]-3,4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63133-84-6 tetramethyl- x Appen1 1(2H)-Quinolinepropanamide, 6-(2,2- dicyanoethenyl)-3, 4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63467-15-2 tetramethyl-N-phenyl- x Appen1 1,1,1,2-Tetrachloro-2,2-bis(4- -

Lubricating Oil Composition and Additive Therefor Having Improved Wear Properties
(19) TZZ ¥_T (11) EP 2 949 738 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 02.12.2015 Bulletin 2015/49 C10M 141/10 (2006.01) (21) Application number: 15170007.7 (22) Date of filing: 31.05.2015 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Lagona, Jason A. GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Richmond, VA 23225 (US) PL PT RO RS SE SI SK SM TR • Loper, John T. Designated Extension States: Richmond, VA 23233 (US) BA ME Designated Validation States: (74) Representative: Dunleavy, Kevin James MA Mendelsohn, Drucker & Dunleavy, P.C. p/o De Vries & Metman (30) Priority: 30.05.2014 US 201462005135 P Overschiestraat 180 01.05.2015 US 201514701899 1062 XK Amsterdam (NL) (71) Applicant: Afton Chemical Corporation Richmond, Virginia 23219 (US) (54) LUBRICATING OIL COMPOSITION AND ADDITIVE THEREFOR HAVING IMPROVED WEAR PROPERTIES (57) A compression-ignited engine lubricant and a compound or anhydride thereof, and optionally, (iv) a method for reducing engine wear. The lubricant includ- nonaromatic dicarboxylic acid or anhydride, wherein the ing, (a) a base oil; (b) oleamide; (c) zinc dihydrocarbyl hydrocarbyl group of the hydrocarbyl-dicarboxylic acid dithiophosphate; and (d) a functionalized dispersant. The or anhydride has a number average molecular weight of functionalized dispersant is made by reacting together greater than 1800 Daltons as determined by gel perme- (i) a hydrocarbyl-dicarboxylic acid or anhydride, (ii) a ation chromatography. polyamine, (iii) a dicarboxylcontaining fused aromatic EP 2 949 738 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 949 738 A1 Description RELATED APPLICATION 5 [0001] This application claims priority to provisional application Serial No. -

Product Specifications Aviation Kerosene JP8
Product Specifications Aviation Kerosene JP8 TEST METHODS (1) PROPERTY UNITS LIMITS ASTM IP STANDARDS STANDARDS Clear, bright and visually free from solid Appearance matter and undissolved water at Visual ambient temperature. In case of dispute it shall be evaluated at 21º C. standard D 156 (2) Saybolt color inform scale D 6045 Total acidity mg KOH/g maximum 0,015 D 3242 IP 354 Aromatics % V/V maximum 25,0 D 1319 (2) IP 156 maximum 26,5 D 6379 IP 436 Sulfur, Total % m/m maximum 0,30 D 129 IP 336 D 1266 D 2622 D 3120 (3) D 4294 (2) D 5453 Sulfur, Mercaptan or % m/m maximum 0,002 D 3227 (2) IP 342 Doctor Test (4) Doctor Negative D 4952 Distillation (5): D 86 (2) (6) IP 123 Initial boiling point report D 2887 IP 406 ºC 10 % V/V recovered maximum 205 D 7345 (7) ºC 20 % V/V recovered report ºC 50 % V/V recovered report ºC 90 % V/V recovered report ºC End point maximum 300 % V/V Residue maximum 1,5 % V/V Loss maximum 1,5 Flash point (8) ºC minimum 38 D 56 IP 170 D 93 (2) D 3828 Density at 15 ºC or kg/m³ 775 a 840 D 1298 IP 160 Density ºAPI at 60 ºF º API 37,0 a 51,0 D 4052 (2) IP 365 D 7777 Freezing point ºC maximum -47 D 2386 (2) IP 16 D 5972 IP 435 D 7153 IP 529 D 7154 IP 528 Viscosity at -20 ºC mm²/s maximum 8.0 D 445 (2) IP 71 Section 1 D 7042 (9) D 7945 Specific energy MJ/kg minimum 42.8 D 3338 IP 12 D 4529 D 4809 (2) Hydrogen content % m/m minimum 13,4 D 3343 D 3701 D 5291 D7171 (2) Smoke point or mm minimum 25,0 D 1322 IP 598 Smoke point and mm minimum 19.0 D 1322 IP 598 Naphthalenes % V/V maximum 3.0 D 1840 Calculated cetane index report D 976 D 4737 Copper corrosion, 2 h at 100 ºC (212 ºF) Class maximum 1b D 130 IP 154 MIL-DTL-83133K 11, July 18, 2018 Page 1of 5 TEST METHODS (1) PROPERTY UNITS LIMITS ASTM IP STANDARDS STANDARDS Thermal stability (JFTOT) (15): D 3241 IP 323 Test temperature tube rating ºC minimum 260 Tube evaluation, one of the following requirements shall be met (16) (17): maximum <3. -

The Influence of Heavy Metals and Organic Matter on Hexavalent
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio della ricerca- Università di Roma La Sapienza 289 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 47, 2016 The Italian Association of Chemical Engineering Online at www.aidic.it/cet Guest Editors: Angelo Chianese, Luca Di Palma, Elisabetta Petrucci, Marco Stoller Copyright © 2016, AIDIC Servizi S.r.l., ISBN 978-88-95608-38-9; ISSN 2283-9216 DOI: 10.3303/CET1647049 The Influence of Heavy Metals and Organic Matter on Hexavalent Chromium Reduction by Nano Zero Valent Iron in Soil Mouhamadou Thierno Gueyea, Luca Di Palmab, Gunel Allahverdeyevac, Irene b b b b Bavasso , Elisabetta Petrucci , Marco Stoller , Giorgio Vilardi a Université Cheikh Anta Diop de Dakar (UCAD), Dakar Fann, Senegal b Sapienza Università di Roma, Dipartimento di Ingegneria Chimica Materiali Ambiente, via Eudossiana 18, 00184 Roma c Baku State University, 23 Khalilov St., Baku, Azerbaijan During the last decades great attention has been payed at evaluating the feasibility of Cr(VI) reduction in soil 0 by nano zero valent iron (nZVI). An inhibitory effect on the Cr(VI) reduction by Fe nanoparticles is generally shown in the presence of high level of heavy metals and natural organic matter in soil. Heavy metals in the environment can react with nZVI by redox reactions, precipitation/dissolution reactions, and adsorption/desorption phenomena. As a result of the presence of metals as Ni, Pb, a decrease in the rate of Cr(VI) reduction was observed. Hence, in the present study, experimental tests of Cr(VI) reduction by nZVI in the presence of selected heavy metals, such as nickel and lead, and in the presence of high level of organic matter, are presented and discussed.