Polymerization of Safflower Oil in a Diesel Lubrication System by John Walter Olson a Thesis Submitted in Partial Fulfillment Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improving Sexual Health: Vaginal Lubricants, Moisturizers, Dilators & Counseling
Improving Sexual Health: Vaginal Lubricants, Moisturizers, Dilators & Counseling Lubricants and moisturizers are effective in relieving pain during intercourse for many midlife women. If you have more severe vaginal dryness and related pain, or if lubricants and moisturizers don’t work well for you, see your health care provider as other options are available. How do vaginal lubricants work? Vaginal lubricants work by reducing the friction associated with thin, dry genital tissue. They come in liquid or gel form and are applied to the vagina and vulva right before intercourse (it can be helpful to apply them to the penis or any body part/instrument inserted into the vagina as well). Lubricants are absorbed into the skin, are immediate-acting, and provide temporary relief from vaginal dryness and related pain during intercourse. Lubricants are available either as water-based, silicone-based, or oil-based products. Water-based lubricants have the advantage of being non-staining and they wash off fairly easily. If you are concerned about staining your sheets, put down a large bath or beach towel on top of your sheets. Do not use oil-based lubricants (such as petroleum jelly and baby oil) because they can cause vaginal irritation. Additionally, oil-based lubricants may make condoms less effective. Some lubricants have glycerin and others are glycerin free. We recommend that you avoid glycerin, flavored lubricants, and warming/”tingling” lubricants. Silicone lubricants are often good options. For a list of lubricants that are available in drug and grocery stores, and online, see pages 4-5 of this handout. What are vaginal moisturizers? Vaginal moisturizers are products that are intended to ease vaginal dryness. -

(Copcs) Recommendations Fuel Additives Red Hill Bulk Fuel Storage Fuel Facility
Chemicals of Potential Concern (COPCs) Recommendations Fuel Additives Red Hill Bulk Fuel Storage Fuel Facility A meeting was held on May 10, 2016 to discuss the recommended approach to addressing the objectives of the Administrative Order on Consent (AOC) In the Matter of Red Hill Bulk Fuel Storage Facility (herein referred to as “the Facility”) Statement of Work (SOW) Section 6 and Section 7 with the Regulatory Agencies (State of Hawaii Department of Health [DOH] and United States Environmental Protection Agency Region IX [EPA]) and various subject matter experts (SMEs). The following attended the meeting: Parties of the AOC (Regulatory Agencies, Department of Navy [Navy], and Defense Logistics Agency [DLA]) and SMEs to the Regulatory Agencies (University of Hawaii [UH]; State of Hawaii Department of Land and Natural Resources [DLNR] Commission on Water Resources Management [COWRM]; United States Geological Survey [USGS] Pacific Islands Water Science Center; City and County of Honolulu Board of Water Supply [BWS]). Also in attendance were the Navy’s contractor, AECOM, and BWS’ contractor, Intera Geoscience & Engineering Solutions (Intera). One of the action items from the meeting was for the Navy and DLA to evaluate fuel additives and determine if additional analytes need to be included on the chemicals of potential concern (COPCs) list for the Facility, as previously agreed upon by the Parties of the AOC on February 4, 2016. The following discussion and table present the results of the fuel additives evaluation: Table 1 summarizes 18 chemical constituents of additives associated with fuel stored at the Facility. Six groups of fuel additives were identified and evaluated: (1) metal deactivators; (2) corrosion inhibitors and lubricity improvers; (3) icing inhibitors; (4) static dissipaters; (5) lubricity improvers; and (6) antioxidants. -

Utilization of Waste Cooking Oil Via Recycling As Biofuel for Diesel Engines
recycling Article Utilization of Waste Cooking Oil via Recycling as Biofuel for Diesel Engines Hoi Nguyen Xa 1, Thanh Nguyen Viet 2, Khanh Nguyen Duc 2 and Vinh Nguyen Duy 3,* 1 University of Fire Fighting and Prevention, Hanoi 100000, Vietnam; [email protected] 2 School of Transportation and Engineering, Hanoi University of Science and Technology, Hanoi 100000, Vietnam; [email protected] (T.N.V.); [email protected] (K.N.D.) 3 Faculty of Vehicle and Energy Engineering, Phenikaa University, Hanoi 100000, Vietnam * Correspondence: [email protected] Received: 16 March 2020; Accepted: 2 June 2020; Published: 8 June 2020 Abstract: In this study, waste cooking oil (WCO) was used to successfully manufacture catalyst cracking biodiesel in the laboratory. This study aims to evaluate and compare the influence of waste cooking oil synthetic diesel (WCOSD) with that of commercial diesel (CD) fuel on an engine’s operating characteristics. The second goal of this study is to compare the engine performance and temperature characteristics of cooling water and lubricant oil under various engine operating conditions of a test engine fueled by waste cooking oil and CD. The results indicated that the engine torque of the engine running with WCOSD dropped from 1.9 Nm to 5.4 Nm at all speeds, and its brake specific fuel consumption (BSFC) dropped at almost every speed. Thus, the thermal brake efficiency (BTE) of the engine fueled by WCOSD was higher at all engine speeds. Also, the engine torque of the WCOSD-fueled engine was lower than the engine torque of the CD-fueled engine at all engine speeds. -

Lubricants in Pharmaceutical Solid Dosage Forms
Lubricants 2014, 2, 21-43; doi:10.3390/lubricants2010021 OPEN ACCESS lubricants ISSN 2075-4442 www.mdpi.com/journal/lubricants Review Lubricants in Pharmaceutical Solid Dosage Forms Jinjiang Li * and Yongmei Wu Drug Product Science & Technology, Bristol-Myers Squibb Corporation, 1 Squibb Dr., New Brunswick, NJ 08903, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-732-227-6584; Fax: +1-732-227-3784. Received: 18 December 2013; in revised form: 21 January 2014 / Accepted: 24 January 2014 / Published: 25 February 2014 Abstract: Lubrication plays a key role in successful manufacturing of pharmaceutical solid dosage forms; lubricants are essential ingredients in robust formulations to achieve this. Although many failures in pharmaceutical manufacturing operations are caused by issues related to lubrication, in general, lubricants do not gain adequate attention in the development of pharmaceutical formulations. In this paper, the fundamental background on lubrication is introduced, in which the relationships between lubrication and friction/adhesion forces are discussed. Then, the application of lubrication in the development of pharmaceutical products and manufacturing processes is discussed with an emphasis on magnesium stearate. In particular, the effect of its hydration state (anhydrate, monohydrate, dihydrate, and trihydrate) and its powder characteristics on lubrication efficiency, as well as product and process performance is summarized. In addition, the impact of lubrication on the dynamics of compaction/compression processes and on the mechanical properties of compacts/tablets is presented. Furthermore, the online monitoring of magnesium stearate in a blending process is briefly mentioned. Finally, the chemical compatibility of active pharmaceutical ingredient (API) with magnesium stearate and its reactive impurities is reviewed with examples from the literature illustrating the various reaction mechanisms involved. -
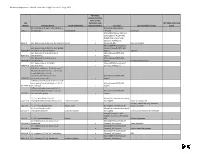
Chemicals of High Concern List (Sorted Alphabetically), July 2010
Minnesota Department of Health, Chemicals of High Concern list, July 1, 2010 Persistent, Bioaccumulative, Toxic or very CAS Persistent, very HPV (2006 and 3 of 4 Number Chemical Name Health endpoint(s) Bioaccumulative Source(s) Use example(s) or class years) (S)-4-hydroxy-3-(3-oxo-1-phenylbutyl)-2- Maine (EU Reproductive 5543-57-7 benzopyrone Reproduction Toxicant) Sunscreen Maine (CA Prop 65; IARC; EU Carcinogen; NTP 11th ROC; OSPAR Chemicals of High Concern); WA Appen1; 91-94-1 [1,1'-biphenyl]-4,4'-diamine, 3,3'-dichloro-Cancer x Minnesota HRL Dye, curing agent Maine (OSPAR Chemicals of [1,1'-biphenyl]-4,4'-diamine, N,N'-bis(2,4- Concern; Canada PBiT); WA 29398-96-7 dinitrophenyl)-3,3'-dimethoxy- x Appen1 Colorant [1,1'-Biphenyl]-4-ol, 3,4',5-tris(1,1- Maine (Canada PBiT); WA 6257-39-2 dimethylethyl)- x Appen1 [1,1'-Biphenyl]-4-ol, 3,4'-bis(1,1- Maine (Canada PBiT); WA 42479-88-9 dimethylethyl)- x Appen1 Chemical intermediate [1,1'-biphenyl]-4-ol, 3,5-bis(1,1- Maine (OSPAR Chemicals of 2668-47-5 dimethylethyl)- x Concern); WA Appen1 [2,6'-Bibenzothiazole]-7-sulfonic acid, 2'- (4-aminophenyl)-6-methyl-, diazotized, coupled with diazotized 4- aminobenzenesulfonic acid and Maine (Canada PBiT); WA 91696-90-1 resorcinol, sodium salts x Appen1 1(2H)-Quinolineethanol, 6-[(2-chloro-4,6- dinitrophenyl) azo]-3,4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63133-84-6 tetramethyl- x Appen1 1(2H)-Quinolinepropanamide, 6-(2,2- dicyanoethenyl)-3, 4-dihydro-2,2,4,7- Maine (Canada PBiT); WA 63467-15-2 tetramethyl-N-phenyl- x Appen1 1,1,1,2-Tetrachloro-2,2-bis(4- -

Product Specifications Aviation Kerosene JP8
Product Specifications Aviation Kerosene JP8 TEST METHODS (1) PROPERTY UNITS LIMITS ASTM IP STANDARDS STANDARDS Clear, bright and visually free from solid Appearance matter and undissolved water at Visual ambient temperature. In case of dispute it shall be evaluated at 21º C. standard D 156 (2) Saybolt color inform scale D 6045 Total acidity mg KOH/g maximum 0,015 D 3242 IP 354 Aromatics % V/V maximum 25,0 D 1319 (2) IP 156 maximum 26,5 D 6379 IP 436 Sulfur, Total % m/m maximum 0,30 D 129 IP 336 D 1266 D 2622 D 3120 (3) D 4294 (2) D 5453 Sulfur, Mercaptan or % m/m maximum 0,002 D 3227 (2) IP 342 Doctor Test (4) Doctor Negative D 4952 Distillation (5): D 86 (2) (6) IP 123 Initial boiling point report D 2887 IP 406 ºC 10 % V/V recovered maximum 205 D 7345 (7) ºC 20 % V/V recovered report ºC 50 % V/V recovered report ºC 90 % V/V recovered report ºC End point maximum 300 % V/V Residue maximum 1,5 % V/V Loss maximum 1,5 Flash point (8) ºC minimum 38 D 56 IP 170 D 93 (2) D 3828 Density at 15 ºC or kg/m³ 775 a 840 D 1298 IP 160 Density ºAPI at 60 ºF º API 37,0 a 51,0 D 4052 (2) IP 365 D 7777 Freezing point ºC maximum -47 D 2386 (2) IP 16 D 5972 IP 435 D 7153 IP 529 D 7154 IP 528 Viscosity at -20 ºC mm²/s maximum 8.0 D 445 (2) IP 71 Section 1 D 7042 (9) D 7945 Specific energy MJ/kg minimum 42.8 D 3338 IP 12 D 4529 D 4809 (2) Hydrogen content % m/m minimum 13,4 D 3343 D 3701 D 5291 D7171 (2) Smoke point or mm minimum 25,0 D 1322 IP 598 Smoke point and mm minimum 19.0 D 1322 IP 598 Naphthalenes % V/V maximum 3.0 D 1840 Calculated cetane index report D 976 D 4737 Copper corrosion, 2 h at 100 ºC (212 ºF) Class maximum 1b D 130 IP 154 MIL-DTL-83133K 11, July 18, 2018 Page 1of 5 TEST METHODS (1) PROPERTY UNITS LIMITS ASTM IP STANDARDS STANDARDS Thermal stability (JFTOT) (15): D 3241 IP 323 Test temperature tube rating ºC minimum 260 Tube evaluation, one of the following requirements shall be met (16) (17): maximum <3. -

Vegetable Oils As Lube Basestocks: a Review
African Journal of Biotechnology Vol. 12(9), pp. 880-891, 27 February, 2013 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB12.2823 ISSN 1684–5315 ©2013 Academic Journals Review Vegetable oils as lube basestocks: A review Anjana Srivastava1* and Preeti Sahai2 1Department of Chemistry, Amity Institute of Applied Sciences, Amity University, UP, India. 2Department of Science and Technology, Technological Development Board, India. Accepted 15 February, 2013 The depletion of the world’s crude oil reserve, increasing crude oil prices, and issues related to conservation have brought about renewed interest in the use of bio-based materials. Emphasis on the development of renewable, biodegradable, and environmentally friendly lubricants has resulted in the widespread use of natural oils and fats. Vegetable oils are promising candidates as base fluid for eco- friendly lubricants because of their excellent lubricity, biodegradability, viscosity-temperature characteristics and low volatility. In view of agriculture based Indian economy, there is a great potential of producing vegetable oil based lubricants, which has ecological compatibility in addition to technical performance. However, suitability of the vegetable oils for a specific application either needs chemical modification or may be used as it is with additive blending route in order to get basestocks as per specifications for a particular end use application. Key words: Biolubricants, vegetable oils, biodegradable, renewable. INTRODUCTION In recent years, deliberate and accidental lubricant losses products because of their environment-friendly and to the environment by means including evaporation, nontoxic nature. Synthetic lubricant base oils are also leakages, and spills have lead to major concerns available and offer improved stability and performance regarding pollution and environmental health. -

Vegetable Oils As Lubricant Base
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 ISSN 2229-5518 708 9HJHWDEOHRLOVDVOXEULFDQWEDVHRLO$5HYLHZ RANI.S, 7$581060/-2<.35$%+$.$5$11$,5 'HSDUWPHQWRI0HFKDQLFDO(QJLQHHULQJ 1DWLRQDO,QVWLWXWHRI7HFKQRORJ\&DOLFXW&DOLFXW Abstract— The demand for industrial lubricants is increasing were based on vegetable oils. In the US around 8,250,000 day by day. Due to the industrial revolution most of the Asian tons/year were consumed in 2002 among which 25,000 countries becomes the manufacturing centers for the world. Lot tons/year were based on vegetables [6]. of used lubricants is thrown to the environment which may damage the environment. In this scenario industry started searching for environmental friendly, renewable and less toxic lubricants . The biolubricant formulation is one of the upcoming research areas in tribology. In this paper the lubricant properties of vegetable oils have been discussed with the available literature. Keywords— environmental friendly; renewable; Biolubricant formulation I. INTRODUCTION Mineral oil and synthetic oil from petrochemical are mainly used as base oil in present lubricant industry. The Fig. 1. Worldwide lubrication consumption in 2004 [4] petroleum resources are diminishing rapidly and the rate of production from older domestic oil fields and decrease in the rate of finding new reservesIJSER are one of the main problems of The renewable and biodegradable vegetable oils have been the 21st century. Petroleum products are poorly degradable [1] used various industrial applications like Chain saw bar and cause severe environmental hazards when released [2] lubricants [7], engine lubricants [8,9], drilling lubricants [10], .The spilling of mineral oil causes negative changes in the metal cutting fluid [11,12], hydraulic fluids [13,14] and sand which affects the agricultural growth a lot [3]. -

Lubricant Additives Lubricant Additives
Worldwide Ltd LUBRICANT ADDITIVES LUBRICANT ADDITIVES VANDERBILT WORLDWIDE Ltd 12 Park House Alvaston Business Park, Middlewich Rd. Nantwich, Cheshire CW5 6PF, UK +44 1270 623978 [email protected] www.vanderbiltworldwide.com VANDERBILT CHEMICALS, LLC CERTIFIED TO ISO 9001:2015 10002461 NSF® Certified Registered and pending trademarks appearing in these materials are those of R.T. Vanderbilt Holding Company, Inc. or its respective wholly owned subsidiaries. For complete listings, please visit this location for trademarks, www.rtvanderbiltholding.com. NSF is a registered trademark of NSF International. UL is a registered trademark of UL LLC. TPS is a registered trademark of Arkema France Corporation Rev. 01/06/2020 DISCLAIMER Before using, read, understand and comply with the information and precautions in the Safety Data Sheets, label and other product literature. The information presented herein, while not guaranteed, was prepared by technical personnel and, to the best of our knowledge and belief, is true and accurate as of the date hereof. No warranty, representation or guarantee, express or implied, is made regarding accuracy, performance, stability, reliability or use. This information is not intended to be all-inclusive, because the manner and conditions of use, handling, storage and other factors may involve other or additional safety or performance considerations. The user is responsible for determining the suitability of any material for a specific purpose and for adopting such safety precautions as may be required. Vanderbilt Chemicals, LLC does not warrant the results to be obtained in using any material, and disclaims all liability with respect to the use, handling or further processing of any such material. -

Glossary of Petrochemical Terms
GLOSSARY OF PETROCHEMICAL TERMS Acid Number: A measure of the amount of potassium hydroxide (KOH) needed to neutralize all or part of the acidity of a petroleum product. Also specified as neutralization number (NN) or value (NV) and total acid number (TAN) Additive: A chemical substance which, when blended with a petroleum product, has the effect of improving one or more of its properties or performance characteristics. Aliphatic Hydrocarbon: Hydrocarbons in which the carbon atoms are arranged in open chains which may be straight or branched. Aniline Point: The minimum temperature for complete miscibility of equal volumes of aniline and the sample under test. Products with high aromatic or naphthenic contents have lower aniline points than products with high paraffinic content. Anti-knock: Resistance of a gasoline (petrol) to detonation in a combustion chamber. API Gravity: A special function of relative density represented by: API Gravity, degrees = 141.5/rel.density at 15.6°C – 131.5 API Service Classification: A system of letter designations agreed by API, SAE and ASTM to define broad classes of engine service. Also used for service classification of automotive gear lubricants. Aromatic: A hydrocarbon derived from, or characterized by, the presence of a benzene ring, or a polymeric (multiple) ring structure. Ash: Non-combustible residue of lubricating oil or fuel,; lubricating oil detergent additives containing metallic derivatives are a common source of ash (see also sulphated ash). Bactericide: An additive to inhibit bacterial growth in aqueous component or phase of fluids, preventing bacterial degradation of the fluid and the resulting foul odours. Base Number: The amount of acid required to neutralize all or part of a lubricant’s basicity, expressed as potassium hydroxide (KOH) equivalents. -

IIIHHHHHHHHHHH USOO5298.178A United States Patent (19) 11 Patent Number: 5,298,178 O'neil Et Al
IIIHHHHHHHHHHH USOO5298.178A United States Patent (19) 11 Patent Number: 5,298,178 O'Neil et al. 45) Date of Patent: Mar. 29, 1994 (54) TRIAZOLE COMPOUNDS USEFUL AS 4,701,273 10/1987 Brady ................................. 252/32.5 4,791,206 12/1988 O'Neil et al. ... 548/108 METAL DEACTIVATORS 4,855,070 8/1989 Lewis .................................... 252/77 75) Inventors: Robert M. O'Neil, Flixton, England; 4,886,611 12/1989 Kapusinski et al. .................. 252/47 Paul Dubs, Marly, Switzerland 4,897,086 l/1990 Blair et al. 4,944,616 8/1990 Falla et al. ............................ 252/77 73) Assignee: Ciba-Geigy Corporation, Ardsley, 4,992, 185 2/1991 Shim ................. ... 252/77 N.Y. 5,021,181 6/1991 Kanamori et al. .................... 252/77 5,032,300 7/1991 O'Neil ...................... 252/.51.5 R (21) Appl. No.: 879,484 5,076,946 12/1991 Frankenfeld et al. ".co- 548/257 22 Filed: May 7, 1992 FOREIGN PATENT DOCUMENTS Related U.S. Application Data 0006710 1/1980 European Pat. Off... 62) Division of Ser. No. 713,943, Jun. 12, 1991, Pat. No. Primary Examiner-Margaret Medley 5,133,890. Attorney, Agent, or Firm-Luther A. R. Hall (30) Foreign Application Priority Data (57) ABSTRACT Jun. 13, 1990 (GB) United Kingdom ................. 9013142 Composition comprising an aqueous or partially aque ous functional fluid susceptible to degradation by a 51) int. Cl. ................ C10M 173/00; C10M 173/02; COM 33/44 metal; and b) a metal deactivator having the formula I: 52 U.S. Cl. .............................. 252/49.3; 252/51.5 R; 252/77 (58) Field of Search ..................... -

Used Oil Re-Refining Study to Address Energy Policy Act of 2005 Section 1838
U.S. Department of Energy • Offi ce of Fossil Energy Used Oil Re-refi ning Study to Address Energy Policy Act of 2005 Section 1838 July 2006 Used Oil Re-refining Study to Address Energy Policy Act of 2005 Section 1838 Office of Oil and Natural Gas Office of Fossil Energy U.S. Department of Energy Used Oil Re-refining Study to Address Energy Policy Act of 2005 Section 1838 Table of Contents 1 Executive Summary........................................................................................... 1-1 1.1 Methodology.............................................................................................. 1-1 1.2 Executive Summary Roadmap.................................................................. 1-1 1.3 Worldwide Lube Oil Demand Perspective and Used Oil Management Practices.................................................................................................... 1-2 1.4 Stakeholders in the U.S. Used Oil Business ............................................. 1-3 1.5 Energy and Environmental Impacts........................................................... 1-4 1.6 Existing Federal and State Regulations Dealing with Used Oils ............... 1-5 1.7 Reusing a Valuable Resource................................................................... 1-6 1.8 Specific Steps That Could be Taken to Improve Collections and Increase Re-refining and Other Beneficial Re-use of Used Oils ............... 1-7 1.8.1 Utilize Information Exchange Processes to Encourage Progress on Used Oil Management and Recycling .................................................