Heliconlus ERATO PHYLLIS (NYMPHALIDAE)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uehara-Prado Marcio D.Pdf
FICHA CATALOGRÁFICA ELABORADA PELA BIBLIOTECA DO INSTITUTO DE BIOLOGIA – UNICAMP Uehara-Prado, Marcio Ue3a Artrópodes terrestres como indicadores biológicos de perturbação antrópica / Marcio Uehara do Prado. – Campinas, SP: [s.n.], 2009. Orientador: André Victor Lucci Freitas. Tese (doutorado) – Universidade Estadual de Campinas, Instituto de Biologia. 1. Indicadores (Biologia). 2. Borboleta . 3. Artrópode epigéico. 4. Mata Atlântica. 5. Cerrados. I. Freitas, André Victor Lucci. II. Universidade Estadual de Campinas. Instituto de Biologia. III. Título. (rcdt/ib) Título em inglês: Terrestrial arthropods as biological indicators of anthropogenic disturbance. Palavras-chave em inglês : Indicators (Biology); Butterflies; Epigaeic arthropod; Mata Atlântica (Brazil); Cerrados. Área de concentração: Ecologia. Titulação: Doutor em Ecologia. Banca examinadora: André Victor Lucci Freitas, Fabio de Oliveira Roque, Paulo Roberto Guimarães Junior, Flavio Antonio Maës dos Santos, Thomas Michael Lewinsohn. Data da defesa : 21/08/2009. Programa de Pós-Graduação: Ecologia. iv Dedico este trabalho ao professor Keith S. Brown Jr. v AGRADECIMENTOS Ao longo dos vários anos da tese, muitas pessoas contribuiram direta ou indiretamente para a sua execução. Gostaria de agradecer nominalmente a todos, mas o espaço e a memória, ambos limitados, não permitem. Fica aqui o meu obrigado geral a todos que me ajudaram de alguma forma. Ao professor André V.L. Freitas, por sempre me incentivar e me apoiar em todos os momentos da tese, e por todo o ensinamento passado ao longo de nossa convivência de uma década. A minha família: Dona Júlia, Bagi e Bete, pelo apoio incondicional. A Cris, por ser essa companheira incrível, sempre cuidando muito bem de mim. A todas as meninas que participaram do projeto original “Artrópodes como indicadores biológicos de perturbação antrópica em Floresta Atlântica”, em especial a Juliana de Oliveira Fernandes, Huang Shi Fang, Mariana Juventina Magrini, Cristiane Matavelli, Tatiane Gisele Alves e Regiane Moreira de Oliveira. -

Aggregate?Pinheiro Et Al
610 Why do the ithomiines (Lepidoptera, Nymphalidae) aggregate?Pinheiro et al. Notes on a butterfly pocket in central Brazil 1 2 3 Carlos E. G. Pinheiro , Ísis Meri Medri & Ana Karina Moreyra Salcedo 1Departamento de Zoologia, Universidade de Brasília – UnB, 70910-900 Brasília-DF, Brasil. [email protected] 2,3Programa de Pós-graduação em Ecologia, Depto. de Ecologia, Universidade de Brasília – UnB, 70910-900 Brasília-DF, Brasil. [email protected] ABSTRACT. Why do the ithomiines (Lepidoptera, Nymphalidae) aggregate? Notes on a butterfly pocket in central Brazil. This study provides information on the species composition and the number of butterflies in different phases of an ithomiine aggregation during the 2004 dry season in central Brazil, and tests some hypotheses concerning the pocket formation. The results obtained suggest that ithomiine pockets constitute primarily an adaptation of butterflies to the adverse climatic conditions of the dry season, such as high temperatures and low air relative humidity, rather than the occurrence of large concentrations of adult food resources (flowers visited for nectar were not found in the pocket site) or defense against visually hunting predators (contrary to the prediction tested, the frequency of butterflies bearing birds beak marks on the wings significantly increased along the period of pocket formation, especially in the case of Mechanitis polymnia, the most abundant species in the pocket). Other hypotheses concerning the pocket formation are also discussed. KEYWORDS. Beak marks; insectivorous birds; ithomiine pockets; Müllerian mimicry. RESUMO. Por que os Ithomiinae (Lepidoptera, Nymphalidae) se agregam? Observações sobre um bolsão de borboletas no Brasil central. Este trabalho apresenta dados sobre a composição de espécies e o número de indivíduos encontrados em diferentes fases de formação de um bolsão de Ithomiinae investigado na estação seca de 2004 em uma floresta de galeria do Brasil central, e testa algumas hipóteses relacionadas à formação do bolsão. -
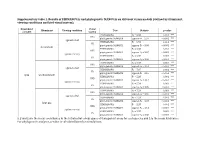
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

TESE DE DOUTORADO � � � � � � � Ricardo Giovenardi � � � � � � � �
0 UNIVERSIDADE FEDERAL DE SANTA MARIA CENTRO DE CIÊNCIAS NATURAIS E EXATAS PROGRAMA DE PÓS-GRADUAÇÃO EM BIODIVERSIDADE ANIMAL COMPOSIÇÃO DE LEPIDOPTERA (PAPILIONOIDEA, HESPERIOIDEA) DO RIO GRANDE DO SUL E VARIABILIDADE ESPAÇO-TEMPORAL EM TRÊS ÁREAS NO NORTE DO ESTADO, BRASIL TESE DE DOUTORADO Ricardo Giovenardi Santa Maria, RS, Brasil 2014 1 COMPOSIÇÃO DE LEPIDOPTERA (PAPILIONOIDEA, HESPERIOIDEA) DO RIO GRANDE DO SUL E VARIABILIDADE ESPAÇO-TEMPORAL EM TRÊS ÁREAS NO NORTE DO ESTADO, BRASIL Ricardo Giovenardi Tese apresentada ao Curso de Doutorado do Programa de Pós-Graduação em Biodiversidade Animal, área de concentração em Bioecologia de Insetos, da Universidade Federal de Santa Maria (UFSM, RS), como requisito parcial para obtenção do grau de Doutor em Biodiversidade Animal. Orientador: Professor Dr. Rocco Alfredo Di Mare Co-orientador: Professor Dr. Olaf Hermann Hendrik Mielke Santa Maria, RS, Brasil 2014 2 3 4 À minha mãe Marilene dos Santos Giovenardi (Leninha) pelo amor incondicional. 5 RESUMO Tese de Doutorado Programa de Pós-Graduação em Biodiversidade Animal Universidade Federal de Santa Maria COMPOSIÇÃO DE LEPIDOPTERA (PAPILIONOIDEA E HESPERIOIDEA) DO RIO GRANDE DO SUL E VARIABILIDADE ESPAÇO-TEMPORAL EM TRÊS ÁREAS NO NORTE DO ESTADO, BRASIL AUTOR: RICARDO GIOVENARDI ORIENTADOR: ROCCO ALFREDO DI MARE COORIENTADOR: OLAF HERMANN HENDRIK MIELKE Data e Local da Defesa: Santa Maria, 12 de setembro de 2014. Com o intuito de contribuir para o conhecimento das borboletas existentes no Rio Grande do Sul, foram consultados trabalhos relacionados com bionomia, taxonomia e inventários florestais, bem como verificou-se a variabilidade espaço-temporal das borboletas em três fragmentos no norte do Estado. Com os estudos acumulados, foi encontrado um total de 832 espécies e subespécies de borboletas. -

Rev Iss Web Mec 13773 25-22 5765..5784
Molecular Ecology (2016) 25, 5765–5784 doi: 10.1111/mec.13773 Into the Andes: multiple independent colonizations drive montane diversity in the Neotropical clearwing butterflies Godyridina NICOLAS CHAZOT,*† KEITH R. WILLMOTT,‡ FABIEN L. CONDAMINE,§¶ DONNA LISA DE-SILVA,* ANDREV.L.FREITAS,**GERARDOLAMAS,†† HELENE MORLON,‡‡ CARLOS E. GIRALDO,§§ CHRIS D. JIGGINS,¶¶ MATHIEU JORON,*** JAMES MALLET,††† SANDRA URIBE‡‡‡ and MARIANNE ELIAS* *Institut de Systematique, Evolution, Biodiversite, ISYEB – UMR 7205 – CNRS MNHN UPMC EPHE, Museum national d’Histoire naturelle, Sorbonne Universites, 57 rue Cuvier CP50, F-75005 Paris, France, †Department of Biology, University of Lund, 223 62 Lund, Sweden, ‡McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA, §CNRS, UMR 5554 Institut des Sciences de l’Evolution (Universite de Montpellier), Place Eugene Bataillon, 34095 Montpellier, France, ¶Department of Biological Sciences, University of Alberta, T6G 2E9 Edmonton, AB, Canada, **Departamento de Zoologia and Museu de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, S~ao Paulo, Brazil, ††Museo de Historia Natural, Universidad Nacional de San Marcos, Lima, Peru, ‡‡IBENS, Ecole Normale Superieure, UMR 8197 CNRS, Paris, France, §§Grupo de Investigacion de Sanidad Vegetal, Universidad Catolica de Oriente, Rionegro, Antioquia, Colombia, ¶¶Department of Zoology, University of Cambridge, Cambridge, UK, ***Centre d’Ecologie Fonctionnelle et Evolutive, CEFE, UMR 5175 CNRS – EPHE – Universite de Montpellier – Universite Paul Valery Montpellier, 34293 Montpellier 5, France, †††Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA, ‡‡‡Universidad Nacional de Colombia, sede Medellın, Medellın, Colombia Abstract Understanding why species richness peaks along the Andes is a fundamental question in the study of Neotropical biodiversity. -

Effects of Land Use on Butterfly (Lepidoptera: Nymphalidae) Abundance and Diversity in the Tropical Coastal Regions of Guyana and Australia
ResearchOnline@JCU This file is part of the following work: Sambhu, Hemchandranauth (2018) Effects of land use on butterfly (Lepidoptera: Nymphalidae) abundance and diversity in the tropical coastal regions of Guyana and Australia. PhD Thesis, James Cook University. Access to this file is available from: https://doi.org/10.25903/5bd8e93df512e Copyright © 2018 Hemchandranauth Sambhu The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owners of any third party copyright material included in this document. If you believe that this is not the case, please email [email protected] EFFECTS OF LAND USE ON BUTTERFLY (LEPIDOPTERA: NYMPHALIDAE) ABUNDANCE AND DIVERSITY IN THE TROPICAL COASTAL REGIONS OF GUYANA AND AUSTRALIA _____________________________________________ By: Hemchandranauth Sambhu B.Sc. (Biology), University of Guyana, Guyana M.Sc. (Res: Plant and Environmental Sciences), University of Warwick, United Kingdom A thesis Prepared for the College of Science and Engineering, in partial fulfillment of the requirements for the degree of Doctor of Philosophy James Cook University February, 2018 DEDICATION ________________________________________________________ I dedicate this thesis to my wife, Alliea, and to our little girl who is yet to make her first appearance in this world. i ACKNOWLEDGEMENTS ________________________________________________________ I would like to thank the Australian Government through their Department of Foreign Affairs and Trade for graciously offering me a scholarship (Australia Aid Award – AusAid) to study in Australia. From the time of my departure from my home country in 2014, Alex Salvador, Katherine Elliott and other members of the AusAid team have always ensured that the highest quality of care was extended to me as a foreign student in a distant land. -

Itapeti E O Seu Entorno
Em razão de sua importância eco- nômica e social para o município de Mogi das Cruzes e do alto grau de degradação que a Serra apre- senta, vários profi ssionais ao longo dos últimos dez anos, trabalharam de forma sistemática para a produ- ção de conhecimentos sobre a sua ocupação, seus aspectos sociais e biológicos. Assim, os capítulos contidos nesse livro representam a compilação de todas as informa- ções com embasamento científi co, de forma a levar o leitor a enten- der um pouco sobre o passado e o presente da Serra do Itapeti e o seu entorno. Itapeti do Serra Serra do VITOR FERNANDES OLIVEIRA DE MIRANDA MARIA SANTINA DE CASTRO MORINI Itapeti Aspectos Históricos, Sociais e Naturalísticos MARIA SANTINA DE CASTRO MORINI VITOR FERNANDES OLIVEIRA DE MIRANDA Serra do Itapeti Aspectos Históricos, Sociais e Naturalísticos Organizadores MARIA SANTINA DE CASTRO MORINI VITOR FERNANDES OLIVEIRA DE MIRANDA 1ª Edição 2012 Rua Machado de Assis, 10-35 Vila América • CEP 17014-038 • Bauru, SP Fone (14) 3313-7968 • www.canal6editora.com.br S4871 Serra do Itapeti: Aspectos Históricos, Sociais e Naturalísticos / Maria Santina de Castro Morini e Vitor Fernandes Oliveira de Miranda (organizadores). - - Bauru, SP: Canal 6, 2012. 400 p. ; 29 cm. ISBN 978-85-7917-174-1 1. Serra do Itapeti. 2. Mata Atlântica. I. Morini, Maria Santina de Castro. II. Miranda, Vitor Fernandes Oliveira de. III. Título. CDD: 577.34 Copyright© Canal6, 2012 Impressão e Acabamento: Av. Dr. Pedro Camarinha, 31 - Santa Cruz do Rio Pardo-SP - T: (14) 3332.1155 - www.graficaviena.com.br PRESERVE A IMPRESSO EM NATUREZA PAPEL RECICLÁVEL Este livro é dedicado .. -

Rezende Luizhenriquegoncalv
UNIVERSIDADE ESTADUAL DE CAMPINAS INSTITUTO DE BIOLOGIA LUIZ HENRIQUE GONÇALVES DE REZENDE DANO FOLIAR AFETA A INTERAÇÃO ENTRE TRICHOGONIOPSIS ADENANTHA (ASTERACEAE) E SEUS VISITANTES FLORAIS? CAMPINAS 2017 LUIZ HENRIQUE GONÇALVES DE REZENDE DANO FOLIAR AFETA A INTERAÇÃO ENTRE TRICHOGONIOPSIS ADENANTHA (ASTERACEAE) E SEUS VISITANTES FLORAIS? Dissertação apresentada ao Instituto de Biologia da Universidade Estadual de Campinas como parte dos requisitos exigidos para a obtenção do título de Mestre em Ecologia. Este arquivo digital corresponde à versão final da Dissertação defendida pelo aluno Luiz Henrique Gonçalves de Rezende e orientada pelo Prof. Dr. Martín Francisco Pareja Piaggio. Orientador: Prof. Dr. Martín Francisco Pareja Piaggio CAMPINAS 2017 Campinas, 12 de julho de 2017. COMISSÃO EXAMINADORA Prof. Dr. Martín Francisco Pareja Piaggio Prof. Dr. Thomas Michael Lewinsohn Prof. Dr. Vinícius Lourenço Garcia de Brito Os membros da Comissão Examinadora acima assinaram a Ata de Defesa, que se encontra no processo de vida acadêmica do aluno. Aos meus queridos avós Euronias e Enelzita Rezende Dirty Paws Jumping up and down the floor My head is an animal And once there was an animal It had a son that mowed the lawn The son was an ok guy They had a pet dragonfly The dragonfly it ran away But it came back with a story to say Her dirty paws and furry coat She ran down the forest slope The forest of talking trees They used to sing about the birds and the bees The bees had declared a war The sky wasn't big enough for them all The birds, they got help from below From dirty paws and the creatures of snow And for a while things were cold They were scared down in their holes The forest that once was green Was colored black by those killing machines But she and her furry friends Took down the queen bee and her men And that's how the story goes The story of the beast with those four dirty paws Of Monsters and Men AGRADECIMENTOS Em primeiro lugar, agradeço a Deus por me trazer até aqui e colocar diante de mim cada experiência que vivi como a oportunidade de crescer. -

Biology and Population Dynamics of Placidula Euryanassa, a Relict Ithomiine Butterfly (Nymphalidae: Ithomiinae)
JOURNAL OF THE LEPIDOPTERISTS' SOCIETY Volume 47 1993 Number 2 Journal of the Lepidopterists' Society 47(2). 1993. 87-105 BIOLOGY AND POPULATION DYNAMICS OF PLACIDULA EURYANASSA, A RELICT ITHOMIINE BUTTERFLY (NYMPHALIDAE: ITHOMIINAE) ANDRE VICTOR LUCCI FREITAS Curso de P6s-Gradua<;ao em Ecologia. Departamento de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, GP. 6109. 13081 Campinas, Sao Paulo, Brazil ABSTRACT. Placidula euryanassa is a primitive ithomiine restricted to the Atlantic coast of South America. Females lay eggs in clusters on Brugmansia suaveolens (Sola naceae). The larvae are gregarious, passing through five instars. Pupation occurs off the host plant; adults emerge after 8 to 14 days. In the laboratory the sex ratio is statistically equal to unity; in field captures the sex ratio is male biased. Individual mobility is low and the population size varies greatly during the year. Adults show a type II survival curve. Many biological features indicate that Placidula is relatively more r-selected than most Ithomiinae. Additional key words: immatures, r-strategist. mark-recapture, Solanaceae. Placidula euryanassa (Felder & Felder) is a member of the subfamily Ithomiinae. The systematic position of the species is uncertain (Brown 1987, Motta 1989). The genus Placidula is monotypic (Fox & Real 1971), with little or no morphological variation observed throughout its geographic distribution in southeastern Brazil and neighboring coun tries (D'Almeida 1938, Fox 1940, 1961, Biezanko 1960a, 1960b, Brown & Mielke 1967, Zikan & Zikan 1968, Fox & Real 1971, Brown 1979). Larvae of Placidula euryanassa have been found on the solanaceous plants Brugmansia suaveolens (Willd.) (=Datura suaveolens and D. arborea), B. candida Pers., Datura stramonium L., D. -

Paulina Aparecida Arce.Pdf
UNIVERSIDADE NOVE JULHO - UNINOVE PROGRAMA DE MESTRADO PROFISSIONAL EM GESTÃO AMBIENTAL E SUSTENTABILIDADE - GeAS PAULINA APARECIDA ARCE BORBOLETAS COMO INDICADORES BIOLÓGICOS DE QUALIDADE DO AR: UM ESTUDO NOS PARQUES URBANOS DA CIDADE DE OSASCO - SP São Paulo 2015 PAULINA APARECIDA ARCE BORBOLETAS COMO INDICADORES BIOLÓGICOS DE QUALIDADE DO AR: UM ESTUDO NOS PARQUES URBANOS DA CIDADE DE OSASCO - SP Dissertação de Mestrado apresentada ao Programa de Pós- Graduação em Administração da Universidade Nove de Julho – UNINOVE, como requisito para a obtenção do grau de Mestre em Gestão Ambiental e Sustentabilidade. Orientador: Prof. Dr. Gustavo Silveira Graudenz Co-Orientadora: Dra. Eliane Tigre Guimarães Sant’Anna SÃO PAULO 2015 Arce, Paulina Aparecida. Borboletas como indicadores biológicos de qualidade do ar: um estudo nos parques urbanos da cidade de Osasco – SP./ Paulina Aparecida Arce. 2015. 122 f. Dissertação (mestrado) – Universidade Nove de Julho - UNINOVE, São Paulo, 2015. Orientador (a): Prof. Dr. Gustavo Silveira Graudenz. 1. Gestão ambiental. 2. Indicadores biológicos. I. Graudenz, Gustavo Silveira. II. Titulo CDU 658:504.06 PAULINA APARECIDA ARCE BORBOLETAS COMO INDICADORES BIOLÓGICOS DE QUALIDADE DO AR: UM ESTUDO NOS PARQUES URBANOS DA CIDADE DE OSASCO - SP Dissertação de Mestrado apresentada ao Programa de Pós- Graduação em Administração da Universidade Nove de Julho – UNINOVE, como requisito para a obtenção do grau de Mestre em Gestão Ambiental e Sustentabilidade, pela Banca Examinadora, formada por: São Paulo, 17 de dezembro de 2014 _____________________________________________________________ Presidente: Prof. Gustavo Silveira Graudenz, Dr. – Orientador, UNINOVE _____________________________________________________________ Membro : Profa. Eliane Tigre Guimarães Sant’Anna, Dra., USP _____________________________________________________________ Membro : Prof. Marcelo Luiz Dias da Silva Gabriel, Dr. -

Butterflies (Lepidoptera: Papilionoidea) from the Campos Rupestres of Serra De São José, Minas Gerais, Brazil
Biota Neotropica 19(3): e20180655, 2019 www.scielo.br/bn ISSN 1676-0611 (online edition) Inventory Butterflies (Lepidoptera: Papilionoidea) from the campos rupestres of Serra de São José, Minas Gerais, Brazil Nathália Ribeiro Henriques1* , Marina do Vale Beirão2, Ello Brasil2 & Tatiana Cornelissen1 1 Universidade Federal de São João del Rei, Departamento de Ciências Naturais, Programa de Pós-Graduação em Ecologia, São João del-Rei, MG, Brasil 2 Universidade Federal de Ouro Preto, Departamento de Biologia Geral, Programa de Pós-Graduação em Ecologia de Biomas Tropicais, Ouro Preto, MG, Brasil *Corresponding author: Nathália Ribeiro Henriques, e-mail: [email protected] HENRIQUES, N. R., BEIRÃO, M. V., BRASIL, E., CORNELISSEN, T. Butterflies (Lepidoptera: Papilionoidea) from the campos rupestres of Serra de São José, Minas Gerais, Brazil. Biota Neotropica. 19(3): e20180655. http://dx.doi.org/10.1590/1676-0611-BN-2018-0655 Abstract: We provide the first inventory of butterfly species (Lepidoptera: Papilionoidea) in Serra de São José, Minas Gerais, Brazil. Serra de São José has elevations ranging from 800 m to 1,400 m above sea level; the butterflies were sampled using traps and entomological nets in seven plots along the altitudinal gradient. We recorded 647 butterflies belonging to 112 species and six families. We also recorded one threatened species and three endemic species for the Cerrado domain, which suggests that Serra de São José is an important refuge for butterfly conservation. Keywords: Cerrado, Conservation, Diversity, Inventory, Species richness, Threatened species. Borboletas (Lepidoptera: Papilionoidea) dos campos rupestres da Serra de São José, Minas Gerais, Brasil Resumo: Fornecemos o primeiro levantamento de espécies de borboletas (Lepidoptera: Papilionoidea) da Serra de São José, Minas Gerais, Brazil. -

Running Head 1 the AGE of BUTTERFLIES REVISITED
bioRxiv preprint doi: https://doi.org/10.1101/259184; this version posted February 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Running head 2 THE AGE OF BUTTERFLIES REVISITED (AND TESTED) 3 Title 4 The Trials and Tribulations of Priors and Posteriors in Bayesian Timing of 5 Divergence Analyses: the Age of Butterflies Revisited. 6 7 Authors 8 NICOLAS CHAZOT1*, NIKLAS WAHLBERG1, ANDRÉ VICTOR LUCCI FREITAS2, 9 CHARLES MITTER3, CONRAD LABANDEIRA3,4, JAE-CHEON SOHN5, RANJIT KUMAR 10 SAHOO6, NOEMY SERAPHIM7, RIENK DE JONG8, MARIA HEIKKILÄ9 11 Affiliations 12 1Department of Biology, Lunds Universitet, Sölvegatan 37, 223 62, Lund, Sweden. 13 2Departamento de Biologia Animal, Instituto de Biologia, Universidade Estadual de 14 Campinas (UNICAMP), Cidade Universitária Zeferino Vaz, Caixa postal 6109, 15 Barão Geraldo 13083-970, Campinas, SP, Brazil. 16 3Department of Entomology, University of Maryland, College Park, MD 20742, U.S.A. 17 4Department of Paleobiology, National Museum of Natural History, Smithsonian 18 Institution, Washington, DC 20013, USA; Department of Entomology and BEES 19 Program, University of Maryland, College Park, MD 20741; and Key Lab of Insect 20 Evolution and Environmental Change, School of Life Sciences, Capital Normal 21 University, Beijing 100048, bioRxiv preprint doi: https://doi.org/10.1101/259184; this version posted February 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.