Aggregate?Pinheiro Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biolphilately Vol-64 No-3
BIOPHILATELY OFFICIAL JOURNAL OF THE BIOLOGY UNIT OF ATA MARCH 2020 VOLUME 69, NUMBER 1 Great fleas have little fleas upon their backs to bite 'em, And little fleas have lesser fleas, and so ad infinitum. —Augustus De Morgan Dr. Indraneil Das Pangolins on Stamps More Inside >> IN THIS ISSUE NEW ISSUES: ARTICLES & ILLUSTRATIONS: From the Editor’s Desk ......................... 1 Botany – Christopher E. Dahle ............ 17 Pangolins on Stamps of the President’s Message .............................. 2 Fungi – Paul A. Mistretta .................... 28 World – Dr. Indraneil Das ..................7 Secretary -Treasurer’s Corner ................ 3 Mammalia – Michael Prince ................ 31 Squeaky Curtain – Frank Jacobs .......... 15 New Members ....................................... 3 Ornithology – Glenn G. Mertz ............. 35 New Plants in the Philatelic News of Note ......................................... 3 Ichthyology – J. Dale Shively .............. 57 Herbarium – Christopher Dahle ....... 23 Women’s Suffrage – Dawn Hamman .... 4 Entomology – Donald Wright, Jr. ........ 59 Rats! ..................................................... 34 Event Calendar ...................................... 6 Paleontology – Michael Kogan ........... 65 New Birds in the Philatelic Wedding Set ........................................ 16 Aviary – Charles E. Braun ............... 51 Glossary ............................................... 72 Biology Reference Websites ................ 69 ii Biophilately March 2020 Vol. 69 (1) BIOPHILATELY BIOLOGY UNIT -

Chemical Defence of the Warningly Coloured Caterpillars of Methona Themisto (Lepidoptera: Nymphalidae: Ithomiinae)
Eur. J. Entomol. 106: 253–259, 2009 http://www.eje.cz/scripts/viewabstract.php?abstract=1449 ISSN 1210-5759 (print), 1802-8829 (online) Chemical defence of the warningly coloured caterpillars of Methona themisto (Lepidoptera: Nymphalidae: Ithomiinae) KAMILA F. MASSUDA and JOSÉ R. TRIGO* Laboratório de Ecologia Química, Departamento de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, CP 6109, 13083-970 Campinas, SP, Brazil Key words. Aposematism, Brunfelsia uniflora, Camponotus crassus, Gallus gallus domesticus, learning, Lycosa erythrognatha, predation, Solanaceae, unpalatability Abstract. The caterpillars of the butterfly Methona themisto (Nymphalidae: Ithomiinae) are conspicuously coloured and feed exclu- sively on Brunfelsia uniflora (Solanaceae), a plant that is rich in secondary plant substances, which suggests the caterpillars are chemically protected against predators. Results of experiments indicate that predators determine the survival of Methona themisto caterpillars in the field and laboratory bioassays that this organism is eaten by ants and spiders but not chicks. Both the conspicuous orange and black striped colouration and chemical compounds of Methona themisto caterpillars seem to be related to protection against predation by visually hunting predators. Chicks ate proportionally more of the cryptically coloured 1st instar caterpillars than of the conspicuously coloured later instar caterpillars. That Methona themisto caterpillars are chemically defended is supported by the activity of the dichloromethanic extract of 5th instars in preventing predation by chicks. Caterpillars of Methona themisto are apo- sematic as they are both (1) unpalatable, and (2) their warning signal is easily recognized by potential predators. Chicks learned to avoid the aposematic 3rd or 5th instar caterpillars after one encounter. Mealworms painted to look like caterpillars were also rejected by chicks that had previously encountered Methona caterpillars. -

Final Report for the University of Nottingham / Operation Wallacea Forest Projects, Honduras 2004
FINAL REPORT for the University of Nottingham / Operation Wallacea forest projects, Honduras 2004 TABLE OF CONTENTS FINAL REPORT FOR THE UNIVERSITY OF NOTTINGHAM / OPERATION WALLACEA FOREST PROJECTS, HONDURAS 2004 .....................................................................................................................................................1 INTRODUCTION AND OVERVIEW ..............................................................................................................................3 List of the projects undertaken in 2004, with scientists’ names .........................................................................4 Forest structure and composition ..................................................................................................................................... 4 Bat diversity and abundance ............................................................................................................................................ 4 Bird diversity, abundance and ecology ............................................................................................................................ 4 Herpetofaunal diversity, abundance and ecology............................................................................................................. 4 Invertebrate diversity, abundance and ecology ................................................................................................................ 4 Primate behaviour........................................................................................................................................................... -

A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ross, Gary Noel, "A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico." (1967). LSU Historical Dissertations and Theses. 1315. https://digitalcommons.lsu.edu/gradschool_disstheses/1315 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-14,010 ROSS, Gary Noel, 1940- A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO. Louisiana State University and Agricultural and Mechanical CoUege, Ph.D., 1967 Entomology University Microfilms, Inc., Ann Arbor, Michigan A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO A D issertation Submitted to the Graduate Faculty of the Louisiana State University and A gricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Gary Noel Ross M.S., Louisiana State University, 196*+ May, 1967 FRONTISPIECE Section of the south wall of the crater of Volcan Santa Marta. May 1965, 5,100 feet. ACKNOWLEDGMENTS Many persons have contributed to and assisted me in the prep aration of this dissertation and I wish to express my sincerest ap preciation to them all. -

Uehara-Prado Marcio D.Pdf
FICHA CATALOGRÁFICA ELABORADA PELA BIBLIOTECA DO INSTITUTO DE BIOLOGIA – UNICAMP Uehara-Prado, Marcio Ue3a Artrópodes terrestres como indicadores biológicos de perturbação antrópica / Marcio Uehara do Prado. – Campinas, SP: [s.n.], 2009. Orientador: André Victor Lucci Freitas. Tese (doutorado) – Universidade Estadual de Campinas, Instituto de Biologia. 1. Indicadores (Biologia). 2. Borboleta . 3. Artrópode epigéico. 4. Mata Atlântica. 5. Cerrados. I. Freitas, André Victor Lucci. II. Universidade Estadual de Campinas. Instituto de Biologia. III. Título. (rcdt/ib) Título em inglês: Terrestrial arthropods as biological indicators of anthropogenic disturbance. Palavras-chave em inglês : Indicators (Biology); Butterflies; Epigaeic arthropod; Mata Atlântica (Brazil); Cerrados. Área de concentração: Ecologia. Titulação: Doutor em Ecologia. Banca examinadora: André Victor Lucci Freitas, Fabio de Oliveira Roque, Paulo Roberto Guimarães Junior, Flavio Antonio Maës dos Santos, Thomas Michael Lewinsohn. Data da defesa : 21/08/2009. Programa de Pós-Graduação: Ecologia. iv Dedico este trabalho ao professor Keith S. Brown Jr. v AGRADECIMENTOS Ao longo dos vários anos da tese, muitas pessoas contribuiram direta ou indiretamente para a sua execução. Gostaria de agradecer nominalmente a todos, mas o espaço e a memória, ambos limitados, não permitem. Fica aqui o meu obrigado geral a todos que me ajudaram de alguma forma. Ao professor André V.L. Freitas, por sempre me incentivar e me apoiar em todos os momentos da tese, e por todo o ensinamento passado ao longo de nossa convivência de uma década. A minha família: Dona Júlia, Bagi e Bete, pelo apoio incondicional. A Cris, por ser essa companheira incrível, sempre cuidando muito bem de mim. A todas as meninas que participaram do projeto original “Artrópodes como indicadores biológicos de perturbação antrópica em Floresta Atlântica”, em especial a Juliana de Oliveira Fernandes, Huang Shi Fang, Mariana Juventina Magrini, Cristiane Matavelli, Tatiane Gisele Alves e Regiane Moreira de Oliveira. -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Preliminary Analysis of the Diurnal Lepidoptera Fauna of the Três Picos State Park, Rio De Janeiro, Brazil, with a Note on Parides Ascanius (Cramer, 1775)
66 TROP. LEPID. RES., 21(2):66-79, 2011 SOARES ET AL.: Butterflies of Três Picos PRELIMINARY ANALYSIS OF THE DIURNAL LEPIDOPTERA FAUNA OF THE TRÊS PICOS STATE PARK, RIO DE JANEIRO, BRAZIL, WITH A NOTE ON PARIDES ASCANIUS (CRAMER, 1775) Alexandre Soares1, Jorge M. S. Bizarro2, Carlos B. Bastos1, Nirton Tangerini1, Nedyson A. Silva1, Alex S. da Silva1 and Gabriel B. Silva1 1Departamento de Entomologia, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista s/n, 20940-040 RIO DE JANEIRO-RJ, Brasil. 2Reserva Ecológica de Guapiaçu, Caixa Postal 98112, 28680-000 CACHOEIRAS DE MACACU-RJ, Brasil. Correspondence to Alexandre Soares: [email protected] Abstract - This paper deals with the butterfly fauna of the Três Picos State Park (PETP) area, Rio de Janeiro State (RJ), Brazil, sampled by an inventory of the entomological collections housed in the Museu Nacional/UFRJ (MNRJ) and a recent field survey at Reserva Ecologica de Guapiaçu (REGUA). The lowland butterfly fauna (up to 600m) is compared for both sites and observations are presented onParides ascanius (Cramer, 1775). Resumo - Apresentam-se dados provisórios sobre a Biodiversidade da fauna de borboletas do Parque Estadual dos Três Picos (PETP), Estado do Rio de Janeiro (RJ), Brasil, inventariada mediante o recurso a dados de etiquetas do acervo da coleção entomológica do Museu Nacional/UFRJ (MNRJ) e uma amostragem de campo executada na Reserva Ecologica de Guapiaçu (REGUA). A riqueza da fauna de borboletas da floresta ombrófila densa de baixada (até 600m) é comparada entre ambas as localidades, registrando-se uma extensão recente da área de ocorrência de Parides ascanius (Cramer, 1775). -
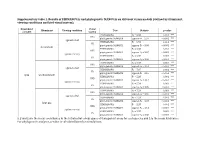
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

BUTTERFLIES in Thewest Indies of the Caribbean
PO Box 9021, Wilmington, DE 19809, USA E-mail: [email protected]@focusonnature.com Phone: Toll-free in USA 1-888-721-3555 oror 302/529-1876302/529-1876 BUTTERFLIES and MOTHS in the West Indies of the Caribbean in Antigua and Barbuda the Bahamas Barbados the Cayman Islands Cuba Dominica the Dominican Republic Guadeloupe Jamaica Montserrat Puerto Rico Saint Lucia Saint Vincent the Virgin Islands and the ABC islands of Aruba, Bonaire, and Curacao Butterflies in the Caribbean exclusively in Trinidad & Tobago are not in this list. Focus On Nature Tours in the Caribbean have been in: January, February, March, April, May, July, and December. Upper right photo: a HISPANIOLAN KING, Anetia jaegeri, photographed during the FONT tour in the Dominican Republic in February 2012. The genus is nearly entirely in West Indian islands, the species is nearly restricted to Hispaniola. This list of Butterflies of the West Indies compiled by Armas Hill Among the butterfly groupings in this list, links to: Swallowtails: family PAPILIONIDAE with the genera: Battus, Papilio, Parides Whites, Yellows, Sulphurs: family PIERIDAE Mimic-whites: subfamily DISMORPHIINAE with the genus: Dismorphia Subfamily PIERINAE withwith thethe genera:genera: Ascia,Ascia, Ganyra,Ganyra, Glutophrissa,Glutophrissa, MeleteMelete Subfamily COLIADINAE with the genera: Abaeis, Anteos, Aphrissa, Eurema, Kricogonia, Nathalis, Phoebis, Pyrisitia, Zerene Gossamer Wings: family LYCAENIDAE Hairstreaks: subfamily THECLINAE with the genera: Allosmaitia, Calycopis, Chlorostrymon, Cyanophrys, -

Rev Iss Web Mec 13773 25-22 5765..5784
Molecular Ecology (2016) 25, 5765–5784 doi: 10.1111/mec.13773 Into the Andes: multiple independent colonizations drive montane diversity in the Neotropical clearwing butterflies Godyridina NICOLAS CHAZOT,*† KEITH R. WILLMOTT,‡ FABIEN L. CONDAMINE,§¶ DONNA LISA DE-SILVA,* ANDREV.L.FREITAS,**GERARDOLAMAS,†† HELENE MORLON,‡‡ CARLOS E. GIRALDO,§§ CHRIS D. JIGGINS,¶¶ MATHIEU JORON,*** JAMES MALLET,††† SANDRA URIBE‡‡‡ and MARIANNE ELIAS* *Institut de Systematique, Evolution, Biodiversite, ISYEB – UMR 7205 – CNRS MNHN UPMC EPHE, Museum national d’Histoire naturelle, Sorbonne Universites, 57 rue Cuvier CP50, F-75005 Paris, France, †Department of Biology, University of Lund, 223 62 Lund, Sweden, ‡McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA, §CNRS, UMR 5554 Institut des Sciences de l’Evolution (Universite de Montpellier), Place Eugene Bataillon, 34095 Montpellier, France, ¶Department of Biological Sciences, University of Alberta, T6G 2E9 Edmonton, AB, Canada, **Departamento de Zoologia and Museu de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, S~ao Paulo, Brazil, ††Museo de Historia Natural, Universidad Nacional de San Marcos, Lima, Peru, ‡‡IBENS, Ecole Normale Superieure, UMR 8197 CNRS, Paris, France, §§Grupo de Investigacion de Sanidad Vegetal, Universidad Catolica de Oriente, Rionegro, Antioquia, Colombia, ¶¶Department of Zoology, University of Cambridge, Cambridge, UK, ***Centre d’Ecologie Fonctionnelle et Evolutive, CEFE, UMR 5175 CNRS – EPHE – Universite de Montpellier – Universite Paul Valery Montpellier, 34293 Montpellier 5, France, †††Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA, ‡‡‡Universidad Nacional de Colombia, sede Medellın, Medellın, Colombia Abstract Understanding why species richness peaks along the Andes is a fundamental question in the study of Neotropical biodiversity. -

1 Universidade Federal Do Rio Grande Do Sul Instituto De
UNIVERSIDADE FEDERAL DO RIO GRANDE DO SUL INSTITUTO DE BIOCIÊNCIAS PROGRAMA DE PÓS-GRADUAÇÃO EM ECOLOGIA Tese de Doutorado Dispersão, processo chave para modular a dinâmica ecológica e evolutiva de borboletas Dirleane Ottonelli Rossato Tese de Doutorado apresentada ao Programa de Pós-Graduação em Ecologia da Universidade Federal do Rio Grande do Sul como um dos pré-requisitos para obtenção do título de Doutora em Ciências – ênfase em Ecologia. Orientador: Prof. Dr. Leandro da Silva Duarte Co-orientador: Prof. Dr. Cristiano Agra Iserhard Porto Alegre, Maio de 2018. 1 Dispersão, processo chave para modular a dinâmica ecológica e evolutiva de borboletas Dirleane Ottonelli Rossato Tese de Doutorado apresentada ao Programa de Pós-Graduação em Ecologia da Universidade Federal do Rio Grande do Sul como um dos pré-requisitos para obtenção do título de Doutora em Ciências – ênfase em Ecologia. Orientador: Prof. Dr. Leandro da Silva Duarte Co-orientador: Prof. Dr. Cristiano Agra Iserhard Banca examinadora: Prof. Dra Helena Piccoli Romanowski Prof. Dr. Thales Renato O. de Freitas Dr. Lucas Jardim Data de defesa pública 28 de maio de 2018. 2 Pés, para que os quero, se tenho asas para voar? Frida Kahlo 3 AGRADECIMENTO Aos orientadores, Professor Leandro Duarte pela orientação, discussão e proposições investigativas tão interessantes envolvendo sistemas ecológicos e evolutivos complexos, Professor Cristiano pela coorientação e suporte na identificação das borboletas. Aos colaboradores de cada um dos capítulos da presente tese, vocês foram essenciais para que esta tese contemplasse diferentes aspectos das causas e efeitos do processo dispersivo de borboletas. Ao Dr. Kaminski pelos seus instigantes questionamentos e interessantes perspectivas sobre a evolução dos caracteres, além da grande colaboração em diversas discussões teóricas e filosóficas relacionadas ou não com a presente tese. -

Sobral-Souza Thadeu M.Pdf
Às famílias Sobral, Souza e Trujillo. AGRADECIMENTOS Agradeço primeiramente aos meus pais pelo dom da vida. Aos meus avós, Elpídio, Iracema, Adilza e Alberto. Sem eles hoje eu não estaria aqui. Agradeço também a minha vózinha Zuleide. A minha mãe, Eunice, pela genética. Contou-me algumas vezes que tentou ser bióloga, mas um bebê chorão, entenda-se “eu”, não a deixou concluir sequer o primeiro semestre. Passados vinte e cinco anos estou aqui tentando realizar nosso maior sonho. Ao meu pai, Cláudio, pelo esforço em tentar entender o meu trabalho. Em “várias” caminhadas na praia discutindo sobre filosofia, ciência e a minha maluquice em estudar borboletas e não fazer um bem para a sociedade! Ah, principalmente, pelo apoio financeiro durante a vida e em algumas viagens, rs. Ao meu irmão, Thiago, pelas brincadeiras, churrascos de recepção após meses no mato, e pela companhia inseparável quando eu toco violão, ou seja, pelo lado bom da vida. Aos meus tios José Marques, Eugênia e aos primos Lucas e Amanda, pelo companheirismo desde criança e pela formação do meu caráter e por me apoiar em todas as decisões. A toda a grandiosa família Souza. Creio eu serem 10 tios, 12 primos e alguns agregados. Se eu fosse escrever o nome de todos iria me esquecer de alguém. Desta forma, agradeço a todos. A minha noiva, Jessica, primeiramente pelo amor a mim dedicado durante todo o tempo. Por entender minhas necessidades (idas semanais ao campo; curso de campo, entre outras coisas). Enfim, por todo o amor, carinho e compreensão depreendida. Tenho certeza que num futuro próximo estará tentando ser uma Ecóloga, assim como eu tento.