Uehara-Prado Marcio D.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sandra Mara Sabedot Bordin
UNIVERSIDADE DO VALE DO RIO DOS SINOS PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA: DIVERSIDADE E MANEJO DA VIDA SILVESTRE SANDRA MARA SABEDOT BORDIN COMPOSIÇÃO E DIVERSIDADE DE BORBOLETAS FRUGÍVORAS (LEPIDOPTERA: NYMPHALIDAE) EM UNIDADES DE CONSERVAÇÃO E FRAGMENTOS FLORESTAIS ADJACENTES DE MATA ATLÂNTICA NO SUL DO BRASIL São Leopoldo 2020 Sandra Mara Sabedot Bordin COMPOSIÇÃO E DIVERSIDADE DE BORBOLETAS FRUGÍVORAS (LEPIDOPTERA: NYMPHALIDAE) EM UNIDADES DE CONSERVAÇÃO E FRAGMENTOS FLORESTAIS ADJACENTES DE MATA ATLÂNTICA NO SUL DO BRASIL Tese apresentada como requisito parcial para a obtenção do título de Doutora em Biologia, área de concentração: Diversidade e Manejo da Vida Silvestre, pela Universidade do Vale do Rio dos Sinos. Orientador: Prof. Dr. Everton Nei Lopes Rodrigues SÃO LEOPOLDO 2020 Ficha catalográfica B729c Bordin, Sandra Mara Sabedot Composição e diversidade de borboletas frugívoras (lepidoptera: nymphalidae) em unidades de conservação e fragmentos florestais adjacentes de mata atlântica no sul do Brasil / Sandra Mara Sabedot. São Leopoldo, RS: 2020. 135 p.: il.; Orientador: Prof. Dr. Everton Nei Lopes Rodrigues Programa de Pós-graduação em Biologia: diversidade e manejo da vida silvestre, 2020. Inclui bibliografias 1. Floresta Ombrófila Mista. 2. Fragmentação. 3. Riqueza de espécies. 4. Inventariamento. 5. Biodiversidade I. Rodrigues, Everton Nei Lopes. II. Título. Catalogação na Fonte Bibliotecária Gabriella Joana CDD: Zorzetto 595.789 CRB 14/1638 i INSTITUIÇÃO EXECUTORA: Universidade do Vale do Rio dos Sinos (UNISINOS) Programa de Pós-Graduação em Biologia: Diversidade e manejo da vida silvestre INSTITUIÇÃO COLABORADORA: Universidade Comunitária da Região de Chapecó (UNOCHAPECÓ) Área de Ciências Exatas e Ambientais Laboratório de Entomologia Ecológica (LABENT-Eco) ii AGRADECIMENTOS A redação de uma tese envolve muito sentimentos e apesar de ser uma atividade individual, a elaboração consiste no envolvimento de muitas outras pessoas. -

Fruit-Feeding Butterflies (Lepidoptera: Nymphalidae) of the Área De
Biota Neotropica 15(3): e20140118, 2015 www.scielo.br/bn inventory Fruit-feeding butterflies (Lepidoptera: Nymphalidae) of the A´ rea de Protec¸a˜o Especial Manancial Mutuca, Nova Lima and Species list for the Region of Belo Horizonte, Minas Gerais, Brazil Andre´ Roberto Melo Silva1,3,4, Douglas Vitor Pontes1, Marco Paulo Guimara˜es1,3, Marina Vicente de Oliveira1, Lucas Tito Faria de Assis1 & Marcio Uehara-Prado2 1Centro Universita´rio UNA, Faculdade de Cieˆncias Biolo´gicas e da Sau´de, Rua Guajajaras, 175, Centro, CEP 30180-100, Belo Horizonte, MG, Brazil. 2Instituto Neotropical: Pesquisa e Conservac¸a˜o Caixa Postal 19009, CEP 81531-980, Curitiba, PR, Brazil. 3Rede de Pesquisa e Conservac¸a˜o de Lepido´pteros de Minas Gerais, Belo Horizonte, MG, Brazil. 4Corresponding author: Andre´ Roberto Melo Silva, e-mail: andrerml.hotmail.com SILVA, A.R.M., PONTES, D.V., GUIMARA˜ ES, M.P., OLIVEIRA, M.V., ASSIS, L.T.F., UEHARA- PRADO, M. Fruit-feeding butterflies (Lepidoptera: Nymphalidae) of the A´ rea de Protec¸a˜o Especial Manancial Mutuca, Nova Lima and Species list for the Region of Belo Horizonte, Minas Gerais, Brazil. Biota Neotropica. 15(3): e20140118. http://dx.doi.org/10.1590/1676-06032015011814 Abstract: A study of the assembly of fruit-feeding butterflies in the A´ rea de Protec¸a˜o Especial Manancial Mutuca, Nova Lima, MG was conducted with the goal of inventorying the species of the site. Forty-two traps were used to attract fruit-feeding butterflies, divided between Cerrado (rupestrian field) and riparian vegetation, monthly over one year. 2245 butterflies, which belonged to 63 species, were recorded. -

Nymphalidae, Brassolinae) from Panama, with Remarks on Larval Food Plants for the Subfamily
Journal of the Lepidopterists' Society 5,3 (4), 1999, 142- 152 EARLY STAGES OF CALICO ILLIONEUS AND C. lDOMENEUS (NYMPHALIDAE, BRASSOLINAE) FROM PANAMA, WITH REMARKS ON LARVAL FOOD PLANTS FOR THE SUBFAMILY. CARLA M. PENZ Department of Invertebrate Zoology, Milwaukee Public Museum, 800 West Wells Street, Milwaukee, Wisconsin 53233, USA , and Curso de P6s-Gradua9ao em Biocicncias, Pontiffcia Universidade Cat61ica do Rio Grande do SuI, Av. Ipiranga 6681, FOlto Alegre, RS 90619-900, BRAZIL ANNETTE AIELLO Smithsonian Tropical Research Institute, Apdo. 2072, Balboa, Ancon, HEPUBLIC OF PANAMA AND ROBERT B. SRYGLEY Smithsonian Tropical Research Institute, Apdo. 2072, Balboa, Ancon, REPUBLIC OF PANAMA, and Department of Zoology, University of Oxford, South Parks Road, Oxford, OX13PS, ENGLAND ABSTRACT, Here we describe the complete life cycle of Galigo illioneus oberon Butler and the mature larva and pupa of C. idomeneus (L.). The mature larva and pupa of each species are illustrated. We also provide a compilation of host records for members of the Brassolinae and briefly address the interaction between these butterflies and their larval food plants, Additional key words: Central America, host records, monocotyledonous plants, larval food plants. The nymphalid subfamily Brassolinae includes METHODS Neotropical species of large body size and crepuscular habits, both as caterpillars and adults (Harrison 1963, Between 25 May and .31 December, 1994 we Casagrande 1979, DeVries 1987, Slygley 1994). Larvae searched for ovipositing female butterflies along generally consume large quantities of plant material to Pipeline Road, Soberania National Park, Panama, mo reach maturity, a behavior that may be related as much tivated by a study on Caligo mating behavior (Srygley to the low nutrient content of their larval food plants & Penz 1999). -

Lepidoptera, Nymphalidae, Biblidinae) and Patterns of Morphological Similarity Among Species from Eight Tribes of Nymphalidae
Revista Brasileira de Entomologia http://dx.doi.org/10.1590/S0085-56262013005000006 External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae Luis Anderson Ribeiro Leite1,2, Mirna Martins Casagrande1,3 & Olaf Hermann Hendrik Mielke1,4 1Departamento de Zoologia, Setor de Ciências Biológicas, Universidade Federal do Paraná, Caixa Postal 19020, 81531–980 Curitiba-PR, Brasil. [email protected], [email protected], [email protected] ABSTRACT. External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae. The external structure of the integument of Dynamine postverta postverta (Cramer, 1779) is based on detailed morphological drawings and scanning electron microscopy. The data are compared with other species belonging to eight tribes of Nymphalidae, to assist future studies on the taxonomy and systematics of Neotropical Biblidinae. KEYWORDS. Abdomen; head; Insecta; morphology; Papilionoidea; thorax. Nymphalidae is a large cosmopolitan family of butter- served in dorsal view (Figs. 1–4). Two subspecies are recog- flies, with about 7,200 described species (Freitas & Brown nized according to Lamas (2004), Dynamine postverta Jr. 2004) and is perhaps the most well documented biologi- postverta (Cramer, 1779) distributed in South America and cally (Harvey 1991; Freitas & Brown Jr. 2004; Wahlberg et Dynamine postverta mexicana d’Almeida, 1952 with a dis- al. 2005). The systematic relationships are still somewhat tribution restricted to Central America. Several species sur- unclear with respect to its subfamilies, tribes and genera, and veys and other studies cite this species as Dynamine mylitta even after more than a century of studies on these groups, (DeVries 1987; Mielke 1994; Miller et al.1999; Freitas & these relationships still seem to confuse many who set out to Brown, Jr. -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Fountainea Centaurus C., R. FELDER, 1867 Y SU POSIBLE RELACIÓN CON Muyshondtia Tyrianthina SALVIN, GODMAN, 1868 (LEPIDOPTERA: NYMPHALIDAE, CHARAXINAE)
Volumen 6 • Número 2 Junio • 2014 Fountainea centaurus C., R. FELDER, 1867 Y SU POSIBLE RELACIÓN CON Muyshondtia tyrianthina SALVIN, GODMAN, 1868 (LEPIDOPTERA: NYMPHALIDAE, CHARAXINAE) Julián A. Salazar-E. Médico Veterinario y Zootecnista, Curador Centro de Museos, Área de Historia Natural, Universidad de Caldas [email protected] Resumen Se compara el patrón de diseño y coloración de dos especies de Charaxinae que habitan la región Andina: Fountainea centaurus C y R Felder, 1867 y Muyshondtia tyrianthina Salvin y Godman, 1868 las cuales presentan una cerrada similitud en el dibujo de sus alas y cierta analogía en sus genitalia respectivas, lo que induce a pensar que M. tyrianthina podría pertenecer al género Fountainea Rydon, 1971. Lo anterior reforzado por el descubrimiento de un macho caudado del Perú que semeja altamente a los machos de F. centaurus. Palabras Clave: Colombia, Charaxinae, Fountainea, Muyshondtia, mimetismo, Perú. Abstract Design pattern and coloration of two species of Neotropical Charaxinae that inhabiting the Andean region: Fountainea centaurus C. y R. Felder, 1867 and Muyshondtia tyrianthina Salvin y Godman, 1868 are compared. Both present a closed similarity in wing pattern and certain analogy in their genitalia, and suggests that M. tyrianthina could belong to the genus Fountainea Rydon, 1971, support by the discovery of a caudate male from Peru, that highly resembles the males of F. centaurus. Key words: Colombia, Charaxinae, Fountainea, Muyshondtia, mimicry, Peru 16 BOLETIN DEL MUSEO ENTOMOLÓGICO FRANCISCO LUÍS GALLEGO Introducción CJS: Colección de Julián Salazar, Manizales Fountainea centaurus Felder y Felder, mejor conocida con su sinónimo de F. CMD: Colección Michael Dottax, Francia nesea Godart, 1824 (Figs. -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

Aggregate?Pinheiro Et Al
610 Why do the ithomiines (Lepidoptera, Nymphalidae) aggregate?Pinheiro et al. Notes on a butterfly pocket in central Brazil 1 2 3 Carlos E. G. Pinheiro , Ísis Meri Medri & Ana Karina Moreyra Salcedo 1Departamento de Zoologia, Universidade de Brasília – UnB, 70910-900 Brasília-DF, Brasil. [email protected] 2,3Programa de Pós-graduação em Ecologia, Depto. de Ecologia, Universidade de Brasília – UnB, 70910-900 Brasília-DF, Brasil. [email protected] ABSTRACT. Why do the ithomiines (Lepidoptera, Nymphalidae) aggregate? Notes on a butterfly pocket in central Brazil. This study provides information on the species composition and the number of butterflies in different phases of an ithomiine aggregation during the 2004 dry season in central Brazil, and tests some hypotheses concerning the pocket formation. The results obtained suggest that ithomiine pockets constitute primarily an adaptation of butterflies to the adverse climatic conditions of the dry season, such as high temperatures and low air relative humidity, rather than the occurrence of large concentrations of adult food resources (flowers visited for nectar were not found in the pocket site) or defense against visually hunting predators (contrary to the prediction tested, the frequency of butterflies bearing birds beak marks on the wings significantly increased along the period of pocket formation, especially in the case of Mechanitis polymnia, the most abundant species in the pocket). Other hypotheses concerning the pocket formation are also discussed. KEYWORDS. Beak marks; insectivorous birds; ithomiine pockets; Müllerian mimicry. RESUMO. Por que os Ithomiinae (Lepidoptera, Nymphalidae) se agregam? Observações sobre um bolsão de borboletas no Brasil central. Este trabalho apresenta dados sobre a composição de espécies e o número de indivíduos encontrados em diferentes fases de formação de um bolsão de Ithomiinae investigado na estação seca de 2004 em uma floresta de galeria do Brasil central, e testa algumas hipóteses relacionadas à formação do bolsão. -

Contributions Toward a Lepidoptera (Psychidae, Yponomeutidae, Sesiidae, Cossidae, Zygaenoidea, Thyrididae, Drepanoidea, Geometro
Contributions Toward a Lepidoptera (Psychidae, Yponomeutidae, Sesiidae, Cossidae, Zygaenoidea, Thyrididae, Drepanoidea, Geometroidea, Mimalonoidea, Bombycoidea, Sphingoidea, & Noctuoidea) Biodiversity Inventory of the University of Florida Natural Area Teaching Lab Hugo L. Kons Jr. Last Update: June 2001 Abstract A systematic check list of 489 species of Lepidoptera collected in the University of Florida Natural Area Teaching Lab is presented, including 464 species in the superfamilies Drepanoidea, Geometroidea, Mimalonoidea, Bombycoidea, Sphingoidea, and Noctuoidea. Taxa recorded in Psychidae, Yponomeutidae, Sesiidae, Cossidae, Zygaenoidea, and Thyrididae are also included. Moth taxa were collected at ultraviolet lights, bait, introduced Bahiagrass (Paspalum notatum), and by netting specimens. A list of taxa recorded feeding on P. notatum is presented. Introduction The University of Florida Natural Area Teaching Laboratory (NATL) contains 40 acres of natural habitats maintained for scientific research, conservation, and teaching purposes. Habitat types present include hammock, upland pine, disturbed open field, cat tail marsh, and shallow pond. An active management plan has been developed for this area, including prescribed burning to restore the upland pine community and establishment of plots to study succession (http://csssrvr.entnem.ufl.edu/~walker/natl.htm). The site is a popular collecting locality for student and scientific collections. The author has done extensive collecting and field work at NATL, and two previous reports have resulted from this work, including: a biodiversity inventory of the butterflies (Lepidoptera: Hesperioidea & Papilionoidea) of NATL (Kons 1999), and an ecological study of Hermeuptychia hermes (F.) and Megisto cymela (Cram.) in NATL habitats (Kons 1998). Other workers have posted NATL check lists for Ichneumonidae, Sphecidae, Tettigoniidae, and Gryllidae (http://csssrvr.entnem.ufl.edu/~walker/insect.htm). -

The Radiation of Satyrini Butterflies (Nymphalidae: Satyrinae): A
Zoological Journal of the Linnean Society, 2011, 161, 64–87. With 8 figures The radiation of Satyrini butterflies (Nymphalidae: Satyrinae): a challenge for phylogenetic methods CARLOS PEÑA1,2*, SÖREN NYLIN1 and NIKLAS WAHLBERG1,3 1Department of Zoology, Stockholm University, 106 91 Stockholm, Sweden 2Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Av. Arenales 1256, Apartado 14-0434, Lima-14, Peru 3Laboratory of Genetics, Department of Biology, University of Turku, 20014 Turku, Finland Received 24 February 2009; accepted for publication 1 September 2009 We have inferred the most comprehensive phylogenetic hypothesis to date of butterflies in the tribe Satyrini. In order to obtain a hypothesis of relationships, we used maximum parsimony and model-based methods with 4435 bp of DNA sequences from mitochondrial and nuclear genes for 179 taxa (130 genera and eight out-groups). We estimated dates of origin and diversification for major clades, and performed a biogeographic analysis using a dispersal–vicariance framework, in order to infer a scenario of the biogeographical history of the group. We found long-branch taxa that affected the accuracy of all three methods. Moreover, different methods produced incongruent phylogenies. We found that Satyrini appeared around 42 Mya in either the Neotropical or the Eastern Palaearctic, Oriental, and/or Indo-Australian regions, and underwent a quick radiation between 32 and 24 Mya, during which time most of its component subtribes originated. Several factors might have been important for the diversification of Satyrini: the ability to feed on grasses; early habitat shift into open, non-forest habitats; and geographic bridges, which permitted dispersal over marine barriers, enabling the geographic expansions of ancestors to new environ- ments that provided opportunities for geographic differentiation, and diversification. -

Preliminary Analysis of the Diurnal Lepidoptera Fauna of the Três Picos State Park, Rio De Janeiro, Brazil, with a Note on Parides Ascanius (Cramer, 1775)
66 TROP. LEPID. RES., 21(2):66-79, 2011 SOARES ET AL.: Butterflies of Três Picos PRELIMINARY ANALYSIS OF THE DIURNAL LEPIDOPTERA FAUNA OF THE TRÊS PICOS STATE PARK, RIO DE JANEIRO, BRAZIL, WITH A NOTE ON PARIDES ASCANIUS (CRAMER, 1775) Alexandre Soares1, Jorge M. S. Bizarro2, Carlos B. Bastos1, Nirton Tangerini1, Nedyson A. Silva1, Alex S. da Silva1 and Gabriel B. Silva1 1Departamento de Entomologia, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista s/n, 20940-040 RIO DE JANEIRO-RJ, Brasil. 2Reserva Ecológica de Guapiaçu, Caixa Postal 98112, 28680-000 CACHOEIRAS DE MACACU-RJ, Brasil. Correspondence to Alexandre Soares: [email protected] Abstract - This paper deals with the butterfly fauna of the Três Picos State Park (PETP) area, Rio de Janeiro State (RJ), Brazil, sampled by an inventory of the entomological collections housed in the Museu Nacional/UFRJ (MNRJ) and a recent field survey at Reserva Ecologica de Guapiaçu (REGUA). The lowland butterfly fauna (up to 600m) is compared for both sites and observations are presented onParides ascanius (Cramer, 1775). Resumo - Apresentam-se dados provisórios sobre a Biodiversidade da fauna de borboletas do Parque Estadual dos Três Picos (PETP), Estado do Rio de Janeiro (RJ), Brasil, inventariada mediante o recurso a dados de etiquetas do acervo da coleção entomológica do Museu Nacional/UFRJ (MNRJ) e uma amostragem de campo executada na Reserva Ecologica de Guapiaçu (REGUA). A riqueza da fauna de borboletas da floresta ombrófila densa de baixada (até 600m) é comparada entre ambas as localidades, registrando-se uma extensão recente da área de ocorrência de Parides ascanius (Cramer, 1775). -
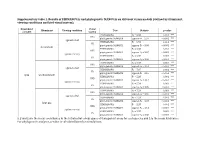
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance.