Quarantine Regulation for Importation of Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download The
9* PSEUDORECOMBINANTS OF CHERRY LEAF ROLL VIRUS by Stephen Michael Haber B.Sc. (Biochem.), University of British Columbia, 1975 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES (The Department of Plant Science) We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA July, 1979 ©. Stephen Michael Haber, 1979 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of Plant Science The University of British Columbia 2075 Wesbrook Place Vancouver, Canada V6T 1W5 Date Jul- 27. 1Q7Q ABSTRACT Cherry leaf roll virus, as a nepovirus with a bipartite genome, can be genetically analysed by comparing the properties of distinct 'parental' strains and the pseudorecombinant isolates generated from them. In the present work, the elderberry (E) and rhubarb (R) strains were each purified and separated into their middle (M) and bottom (B) components by sucrose gradient centrifugation followed by near- equilibrium banding in cesium chloride. RNA was extracted from the the separated components by treatment with a dissociation buffer followed by sucrose gradient centrifugation. Extracted M-RNA of E-strain and B-RNA of R-strain were mixed and inoculated to a series of test plants as were M-RNA of R-strain and B-RNA of E-strain. -

Groundnut Viral Diseases in West Africa
I 1 .. I? ?' GROUNDNUT VIRAL DISEASES IN WEST AFRICA DOLLET Michel*, DUBERN Jean**, FAUQUET Claude***, THOUVENEL Jean-Claude***,and BOCKELEE-MORVAN André**** Respectively : *IRHO/CIRAD, **ORSTOM/CIRAD, BP 5035, 34032 Montpellier, France. *** ORSTOM, BP V 51, Abidjan, Côte d'Ivoire ; all members of the LPRC (CIRAD/ INRA/ORSTOM), Laboratoire de Phytovirologie des Régions Chaudes. **** Division Oléagineux Annuels, IRHO/CIRAD, 11 Square Pétrarque, 75116 Paris', France. Introduction . The groundnut which is one of the most popular legume grown in West Africa, is naturally infected by a Large number of virus. or virus-like diseases. It is one of the most severely-infected tropical plants in number of viral diseases. Some of them were studied and viruses identified : Peanut clump virus (Thouvenel et al., 19761, Groundnut eye spot virys (Dubern' and Dollet, 19801, Groundnut crinkle virus (Dubern and Dollet, 19811, Groundnut rosette virus (Dubern, 19801, Tomato spotted wiIt virus (Dubern and Fauquet, 1985) and Groundnut chlorotic spotting virus (Fauquet et al., 19851. The most important properties according to differe'nt aspects are reported.' Some other diseases are only described in parts : Groundnut streak (Fauquet and Thouvenel, 19-35), Groundnut mosaic, Groundnut f Lecking and Groundnut golden diseases (Dubern, 1979). There are also , different symptoms!wKich could be attributed to viral diseases as rugose leaf, Groundnut leaf-curl, Groundnut bushy stunt ; their etiology is at present unknown. 9 t 2 6 - Peanut clump virus Peanut Clump Virus (PCV), R/1: 2.1/4: E/E: S/Fu. Intermediate between ,hordeivirus and tobamovirus groups (Furovirus ?l. Main disease and 'geographical distribution The disease reappears in the same place in succeeding crops. -

Groundnut Rosette Disease and Their Diagnosis a F Murant, D J Robinson, and M E Taliansky 5
Citation: Reddy, D.V.R., Delfosse, P., Lenne, J.M., and Subrahmanyam, P. (eds.) 1997. Groundnut virus diseases in Africa: summary and recommendations of the Sixth Meeting of the International Working Group, 18-19 Mar 1996, Agricultural Research Council, Plant Protection Research Institute, Pretoria, South Africa. (In En. Summaries in En, Fr, Pt) Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics; and 1000 Brussels, Belgium: Belgian Administration for Development Cooperation. 64 pp. ISBN 92-9066-358-8. Order code: CPE 109. Abstract The International Working Group Meeting on groundnut viruses in Africa reviewed progress made on the detection, identification, characterization, and management of groundnut viruses in Africa, with special emphasis on rosette and clump viruses. Country representatives summarized the status of research on groundnut viruses in their countries. In order to accomplish integrated management of rosette and clump virus diseases, it was agreed that consolidated efforts should be made to understand their epidemiology. Among the important aspects discussed were the provision of diagnostic aids and training in the identifi cation and detection of viruses for the national agricultural research systems in Africa, and strengthening of laboratory facilities. Scientists from Burkina Faso, Kenya, Malawi, Nigeria, South Africa, and Zimbabwe, and from Bel gium, Germany, India, UK, and USA attended the meeting, which was the first gathering of so many plant virologists in -

Sequence Diversity Within the Three Agents of Groundnut Rosette Disease
Virology Sequence Diversity Within the Three Agents of Groundnut Rosette Disease C. M. Deom, R. A. Naidu, A. J. Chiyembekeza, B. R. Ntare, and P. Subrahmanyam First author: Department of Plant Pathology, Plant Sciences Building, The University of Georgia, Athens 30602-7274; second and fifth authors: Genetic Resources and Enhancement Program (GREP), International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), P.O. Box 1096, Lilongwe, Malawi; third author: Department of Agricultural Research and Technical Services, Chitedze Agricultural Research Station, Lilongwe, Malawi; fourth author: GREP, ICRISAT, P.O. Box 320, Bamako, Mali. Current address of R. A. Naidu: Department of Plant Pathology, The University of Georgia, Athens 30602-7274. Accepted for publication 5 December 1999. ABSTRACT Deom, C. M., Naidu, R. A., Chiyembekeza, A. J., Ntare, B. R., and isolates within a geographic region but less conserved (88 to 89%) be- Subrahmanyam, P. 2000. Sequence diversity within the three agents of tween isolates from the two distinct geographic regions. Phylogenetic groundnut rosette disease. Phytopathology 90:214-219. analysis of the overlapping ORFs 3 and 4 show that the GRV isolates cluster according to the geographic region from which they were iso- Sequence diversity was examined in the coat protein (CP) gene of lated, indicating that Malawian GRV isolates are distinct from Nigerian Groundnut rosette assistor virus (GRAV), the overlapping open reading GRV isolates. Similarity within the sat-RNA sequences analyzed ranged frames (ORFs) 3 and 4 of Groundnut rosette virus (GRV), and the satel- from 88 to 99%. Phylogenetic analysis also showed clustering within the lite RNA (sat-RNA) of GRV obtained from field isolates from Malawi sat-RNA isolates according to country of origin, as well as within isolates and Nigeria. -

NBPGR Okf"Kzd Izfrosnu ANNUAL REPORT 2012-2013
ISSN NO 0971-2572 NBPGR okf"kZd izfrosnu ANNUAL REPORT 2012-2013 jk"Vªh; ikni vkuqOakf'kd Laklk/u C;wjks (Hkkjrh; Ñf"k vuqLak/ku ifj"kn) iwlk ifjlj] ubZ fnYyh&110 012 NATIONAL BUREAU OF PLANT GENETIC RESOURCES (Indian Council of Agricultural Research) Pusa Campus, New Delhi - 110 012 Citation : Anonymous (2013). Annual Report of the National Bureau of Plant Genetic Resources 2012-2013, NBPGR, Pusa Campus, New Delhi, India, 186+vi p. Compiled and Edited by : Dr. Arjun Lal, Principal Scientist Dr. (Mrs.) Kavita Gupta, Principal Scientist Dr. (Mrs.) Vandana Tyagi, Principal Scientist Dr. (Mrs.) Sangita Yadav, Senior Scientist This report includes unprocessed or semi-processed data, which would form the basis of scientific papers in due course. The material contained in the report therefore may not be made use of without the written permission of the Director, National Bureau of Plant Genetic Resources, New Delhi except for quoting it for scientific reference. Published by the Director, National Bureau of Plant Genetic Resources, Pusa Campus, New Delhi-110 012, and Printed at Alpha Printographics (India), New Delhi-110 028. Tel.: 9999039940, 9811199620 CONTENTS Preface Executive Summary 1 Introduction 9 NBPGR Headquarters, New Delhi 1. Plant Exploration and Germplasm Collection 13 2. Germplasm Evaluation 19 3. Germplasm Conservation 40 4. Plant Quarantine 45 5. Germplasm Exchange 53 6. Tissue Culture and Cryopreservation 60 7. PGR Policy Planning 65 8. Agricultural Knowledge Management 67 9. Genomic Resources 70 NBPGR Regional Stations/ Base Centers 10. Regional Station, Akola 86 11. Regional Station, Bhowali 91 12. Base Center, Cuttack 96 13. -

Epidemiology of Groundnut Rosette Virus at Samaru, Northern Guinea Savanna of Nigeria
NIGBRIAN JOURNAL OJ' BNTOMOLOGY (2001) 18:95-102 Epidemiology of Groundnut Rosette Virus at Samaru, Northern Guinea Savanna of Nigeria. M. D. ALEGBEJO Department of Crop Protection, Institute for Agricultural.Research/Faculty of Agriculture, Ahmadu Bello University, P.M.B. 1044 Zaria, Nigeria. (A~pted 05 July, 2001) ABSTRACT Epidemiology of groundnut rosette virus (GRV) was investigated over a three - year period at Samaru, Northern Guinea Savanna of Nigeria. Significant positive correlations were obtained for the following: incidence of GRV andnumber of alate aphids trapped; temperature and age of plants; temperature and number of alate aphids trapped; relative humidity and incidence of GRV. Conversely, a significant negative correlation was obtained between age of plants and' number of alate aphids trapped; relative humidity and number of alate aphids trapped; and relative humidity and sunshine hours. Seventy percent of the aphids trapped were A. craccivora, while the proportion of A. jabae, A. gossypii and M. persicae were 15, 9 and 6%, respectively. The susceptible groundnut genotype, 48-1158, had the highest incidence of aphids and GRV disease, followed in descending order by the moderately resistant RRB and the resistant RMP91 genotypes. INTRODUCTION Groundnut rosette virus (GRV) is the most important factor limiting the production ofgroundnut (Arachis hypogaea L.) in Africa especially in Nigeria (Brunt and Bonney, 1964; Rossel, 1977; Alegbejo, 1997). It is transmitted persistently by Aphis craccivora Koch (Rossel, 1997; Misari et al., 1988), My'lJJS persicae Sulzer and Aphis gossypii Glover (Todd et al., 1993; Alegbejo, 2000a). An epidemic of the disease occurred in Nigeria in 1975 and destroyed an estimated 0.7 million hectares of groundnut (Yayock et al., 1976). -

Genomic Characterization of the Cacao Swollen Shoot Virus Complex and Other Theobroma Cacao-Infecting Badnaviruses
Genomic Characterization of the Cacao Swollen Shoot Virus Complex and other Theobroma Cacao-Infecting Badnaviruses Item Type text; Electronic Dissertation Authors Chingandu, Nomatter Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 29/09/2021 07:25:04 Link to Item http://hdl.handle.net/10150/621859 GENOMIC CHARACTERIZATION OF THE CACAO SWOLLEN SHOOT VIRUS COMPLEX AND OTHER THEOBROMA CACAO-INFECTING BADNAVIRUSES by Nomatter Chingandu __________________________ A Dissertation Submitted to the Faculty of the SCHOOL OF PLANT SCIENCES In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY WITH A MAJOR IN PLANT PATHOLOGY In the Graduate College THE UNIVERSITY OF ARIZONA 2016 1 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Nomatter Chingandu, entitled “Genomic characterization of the Cacao swollen shoot virus complex and other Theobroma cacao-infecting badnaviruses” and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy. _______________________________________________________ Date: 7.27.2016 Dr. Judith K. Brown _______________________________________________________ Date: 7.27.2016 Dr. Zhongguo Xiong _______________________________________________________ Date: 7.27.2016 Dr. Peter J. Cotty _______________________________________________________ Date: 7.27.2016 Dr. Barry M. Pryor _______________________________________________________ Date: 7.27.2016 Dr. Marc J. Orbach Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. -

DISEASE RESISTANCE TESTING in COCOA a Review on Behalf of FAO/INGENIC J.C. Zadoks, Consultant
Zadoks - Disease resistance in cocoa 1997 February 2, 1997 DISEASE RESISTANCE TESTING IN COCOA A review on behalf of FAO/INGENIC J.C. Zadoks, consultant do Department of Phytopathology P.O.Box 8025 6700 EE Wageningen The Netherlands private fax + 31 - 317 - 48 34 12 fax + 31 - 317 - 42 36 14 e-mail Jan Carel [email protected] [email protected] - (hoi o Zadoks - Disease resistance in cocoa 1997 TABLE OF CONTENTS 0. Executive summary 1. Introduction 1.1. INGENIC 1.2. Objectives of the review 1.3. Procedure 2. Background information. The host plant 2.1. Origin 2.2. Agronomy 2.3. Genetics 2.4. Breeding 2.5. Pre-breeding 2.6. Conclusions and recommendations 3. Background information. The pathogens 3.1. Taxonom ic cons iderations 3.2. Subspecific variation 3.3. Selected pathogens 3.4. General comments 4. Resistance testing 4.1. Resistance, tolerance and escape 4.2. Components of resistance 4.3. Host-parasite interactions 4.4. Methods of resistance testing 4.5. Predictive tests 4.6. Resistance tests in campo 4.7. Resistance tests in vivo 4.8. Biochemical and molecular tests 4.9. International aspects of resistance testing 5. Resistance 5.1. Past and present situation of resistance breeding 5.2. Breeding objectives 5.3. Results with selected pathogens 5.4. Molecular techniques 5.5. General comments 5.6. Conclusions 2 Zadoks - Disease resistance in cocoa 1997 6. International cooperation 6.1. Organizational aspects 6.2. Needs 6.3. Pre-breeding 6.4. Preliminaries to implementation 6.5. Quality standards 6.6. Recommendations 7. -

International Plant Genetic Resources Instit., (IPGRI)
FAO/IPGRINo. 20. Cacao Technical Guidelines for the Safe Movement of Germplasm No. 201 Cacao edited by E.A. Frison, M. Diekman and D. Nowell netic t Ge Res lan ou P rc al e n s o I ti n a s t n i r t u e t t e n I IPGRI in collaboration with the American Cocoa Research Institute No. 20. Cacao 2 Previously published Technical Guidelines for the Safe Movement of Germplasm These guidelines describe technical procedures that minimize the rist of pest introduc- tions with movement of germplasm for research, crop improvement, plant breeding, exploration or conservation. The recommendations and information in these guide- lines are intended for germplasm for research, conservation and plant breeding programmes. Recommendations for commercial consignments are not the objective of these guidelines. Cocoa 1989 Edible aroids 1989 Musa (1st edition) 1989 Sweet potato 1989 Yam 1989 Legumes 1990 Cassava 1991 Citrus 1991 Grapevine 1991 Vanilla 1991 Coconut 1993 Sugarcane 1993 Small fruits (Fragaria, Ribes, Rubus, Vaccinium) 1994 Musa spp. (2nd edition) 1996 Stone Fruits 1996 Eucalyptus spp. 1996 Allium 1998 Potato 1998 No. 20. Cacao 3 CONTENTS Introduction ................................................ 4 Contributors ............................................... 6 Intermediate quarantine ........................... 8 Stations available for cacao ................ 8 General recommendations ....................... 9 Options for the movement of cacao germplasm in relation to the risk of moving pests ............................................ 10 Descriptions of pests ............................... 12 Viruses ................................................. 12 Cacao necrosis nepovirus ............. 12 Cacao swollen shoot badnavirus . 13 Cacao yellow mosaic lymovirus.. 16 Other virus-like diseases ............ 16 Fungi .................................................... 17 Witches’ broom ............................. 17 Moniliophthora pod rot ................. 19 Vascular streak dieback ............... 21 Phytophthora spp. -

Ajay Kumar Tiwari Editor Advances in Seed Production and Management Advances in Seed Production and Management Ajay Kumar Tiwari Editor
Ajay Kumar Tiwari Editor Advances in Seed Production and Management Advances in Seed Production and Management Ajay Kumar Tiwari Editor Advances in Seed Production and Management Editor Ajay Kumar Tiwari UP Council of Sugarcane Research Shahjahanpur, Uttar Pradesh, India ISBN 978-981-15-4197-1 ISBN 978-981-15-4198-8 (eBook) https://doi.org/10.1007/978-981-15-4198-8 # Springer Nature Singapore Pte Ltd. 2020 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, expressed or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. This Springer imprint is published by the registered company Springer Nature Singapore Pte Ltd. -

List of Viruses Presenting at the Wild State a Biological Risk for Plants
December 2008 List of viruses presenting at the wild state a biological risk for plants CR Species 2 Abutilon mosaic virus 2 Abutilon yellows virus 2 Aconitum latent virus 2 African cassava mosaic virus 2 Ageratum yellow vein virus 2 Agropyron mosaic virus 2 Ahlum waterborne virus 2 Alfalfa cryptic virus 1 2 Alfalfa mosaic virus 2 Alsike clover vein mosaic virus 2 Alstroemeria mosaic virus 2 Amaranthus leaf mottle virus 2 American hop latent virus ( ← Hop American latent virus) 2 American plum line pattern virus 2 Anthoxanthum latent blanching virus 2 Anthriscus yellows virus 2 Apple chlorotic leaf spot virus 2 Apple mosaic virus 2 Apple stem grooving virus 2 Apple stem pitting virus 2 Arabis mosaic virus satellite RNA 2 Araujia mosaic virus 2 Arracacha virus A 2 Artichoke Italian latent virus 2 Artichoke latent virus 2 Artichoke mottled crinkle virus 2 Artichoke yellow ringspot virus 2 Asparagus virus 1 2 Asparagus virus 2 2 Avocado sunblotch viroid 2 Bajra streak virus 2 Bamboo mosaic virus 2 Banana bract mosaic virus 2 Banana bunchy top virus 2 Banana streak virus 2 Barley mild mosaic virus page 1 December 2008 2 Barley mosaic virus 3 Barley stripe mosaic virus 2 Barley yellow dwarf virus-GPV 2 Barley yellow dwarf virus-MAV 2 Barley yellow dwarf virus-PAV 2 Barley yellow dwarf virus-RGV 2 Barley yellow dwarf virus-RMV 2 Barley yellow dwarf virus-SGV 2 Barley yellow mosaic virus 2 Barley yellow streak mosaic virus 2 Barley yellow striate mosaic virus 2 Bean calico mosaic virus 2 Bean common mosaic necrosis virus 2 Bean common mosaic -
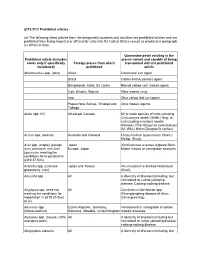
319.37-2 Prohibited Articles
§319.37-2 Prohibited articles. (a) The following listed articles from the designated countries and localities are prohibited articles and are prohibited from being imported or offered for entry into the United States except as provided in paragraph (c) of this section. Quarantine pests existing in the Prohibited article (includes places named and capable of being seeds only if specifically Foreign places from which transported with the prohibited mentioned) prohibited article Abelmoschus spp. (okra) Africa Cotton leaf curl agent. Brazil Cotton Anthocyanosis agent. Bangladesh, India, Sri Lanka Bhendi yellow vein mosaic agent. Cote d'Ivoire, Nigeria Okra mosaic virus. Iraq Okra yellow leaf curl agent. Papua New Guinea, Trinidad and Okra mosaic agents. Tobago Abies spp. (fir) All except Canada 50 or more species of rusts including Chrysomyxa abietis (Wallr.) Ung. (a rust causing a serious needle disease); Phacidiopycnis pseudotsuga (M. Wils.) Hahn (Douglas fir canker). Acacia spp. (acacia) Australia and Oceania Uromycladium tepperianum (Sacc.) McAlp. (Rust). Acer spp. (maple) (except Japan Xanthomonas acernea (Ogawa) Burk. Acer palmatum and Acer Europe, Japan Maple mosaic or variegation diseases. japonicum meeting the conditions for importation in §319.37-5(m) Actinidia spp. (Chinese Japan and Taiwan Pucciniastrum actinidiae Hiratusuka gooseberry, kiwi). (Rust). Adonidia spp All A diversity of diseases including, but not limited to: Lethal yellowing disease; Cadang-cadang disease. Aeglopsis spp. seed not All Candidatus Liberibacter spp. meeting the conditions for (Huanglongbing disease of citrus, importation in §319.37-5(w) Citrus greening). or (x). Aesculus spp. Czech Republic, Germany, Horsechestnut variegation or yellow (horsechestnut) Romania, Slovakia, United Kingdom mosaic diseases.