Vapor Pressure Formulation for Ice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
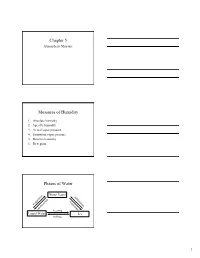
Chapter 5 Measures of Humidity Phases of Water
Chapter 5 Atmospheric Moisture Measures of Humidity 1. Absolute humidity 2. Specific humidity 3. Actual vapor pressure 4. Saturation vapor pressure 5. Relative humidity 6. Dew point Phases of Water Water Vapor n o su ti b ra li o n m p io d a a at ep t v s o io e en s n d it n io o n c freezing Liquid Water Ice melting 1 Coexistence of Water & Vapor • Even below the boiling point, some water molecules leave the liquid (evaporation). • Similarly, some water molecules from the air enter the liquid (condense). • The behavior happens over ice too (sublimation and condensation). Saturation • If we cap the air over the water, then more and more water molecules will enter the air until saturation is reached. • At saturation there is a balance between the number of water molecules leaving the liquid and entering it. • Saturation can occur over ice too. Hydrologic Cycle 2 Air Parcel • Enclose a volume of air in an imaginary thin elastic container, which we will call an air parcel. • It contains oxygen, nitrogen, water vapor, and other molecules in the air. 1. Absolute Humidity Mass of water vapor Absolute humidity = Volume of air The absolute humidity changes with the volume of the parcel, which can change with temperature or pressure. 2. Specific Humidity Mass of water vapor Specific humidity = Total mass of air The specific humidity does not change with parcel volume. 3 Specific Humidity vs. Latitude • The highest specific humidities are observed in the tropics and the lowest values in the polar regions. -

Physics, Chapter 17: the Phases of Matter
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Robert Katz Publications Research Papers in Physics and Astronomy 1-1958 Physics, Chapter 17: The Phases of Matter Henry Semat City College of New York Robert Katz University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/physicskatz Part of the Physics Commons Semat, Henry and Katz, Robert, "Physics, Chapter 17: The Phases of Matter" (1958). Robert Katz Publications. 165. https://digitalcommons.unl.edu/physicskatz/165 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Robert Katz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 17 The Phases of Matter 17-1 Phases of a Substance A substance which has a definite chemical composition can exist in one or more phases, such as the vapor phase, the liquid phase, or the solid phase. When two or more such phases are in equilibrium at any given temperature and pressure, there are always surfaces of separation between the two phases. In the solid phase a pure substance generally exhibits a well-defined crystal structure in which the atoms or molecules of the substance are arranged in a repetitive lattice. Many substances are known to exist in several different solid phases at different conditions of temperature and pressure. These solid phases differ in their crystal structure. Thus ice is known to have six different solid phases, while sulphur has four different solid phases. -

Genesis and Geographical Aspects of Glaciers - Vladimir M
HYDROLOGICAL CYCLE – Vol. IV - Genesis and Geographical Aspects of Glaciers - Vladimir M. Kotlyakov GENESIS AND GEOGRAPHICAL ASPECTS OF GLACIERS Vladimir M. Kotlyakov Institute of Geography, Russian Academy of Sciences, Moscow, Russia Keywords: Chionosphere, cryosphere, equilibrium line, firn line, glacial climate, glacier, glacierization, glaciosphere, ice, seasonal snow line, snow line, snow-patch Contents 1. Introduction 2. Properties of natural ice 3. Cryosphere, glaciosphere, chionosphere 4. Snow-patches and glaciers 5. Basic boundary levels of snow and ice 6. Measures of glacierization 7. Occurrence of glaciers 8. Present-day glacierization of the Arctic Glossary Bibliography Biographical Sketch Summary There exist ten crystal variants of ice and one amorphous form in Nature, however only one form ice-1 is distributed on the Earth. Ten other ice variants steadily exist only under a certain combinations of pressure, specific volume and temperature of medium, and those are not typical for our planet. The ice density is less than that of water by 9%, and owing to this water reservoirs are never totally frozen., Thus life is sustained in them during the winter time. As a rule, ice is much cleaner than water, and specific gas-ice compounds called as crystalline hydrates are found in ice. Among the different spheres surrounding our globe there are cryosphere (sphere of the cold), glaciosphere (sphere of snow and ice) and chionosphere (that part of the troposphere where the annual amount of solid precipitation exceeds their losses). The chionosphere envelopes the Earth with a shell 3 to 5 km in thickness. In the present epoch, snow and ice cover 14.2% of the planet’s surface and more than half of the land surface. -

Pressure Vs Temperature (Boiling Point)
Boiling Points and Vapor Pressure Background Boiling Points and Vapor Pressure Background: Definitions Vapor Pressure: The equilibrium pressure of a vapor above its liquid; the pressure of the vapor resulting from the evaporation of a liquid above a sample of the liquid in a closed container. Boiling Point: The temperature at which the vapor pressure of a liquid is equal to the atmospheric (or applied) pressure. As the temperature of the liquid increases, the vapor pressure will increase, as seen below: https://www.chem.purdue.edu/gchelp/liquids/vpress.html Vapor pressure is interpreted in terms of molecules of liquid converting to the gaseous phase and escaping into the empty space above the liquid. In order for the molecules to escape, the intermolecular forces (Van der Waals, dipole- dipole, and hydrogen bonding) have to be overcome, which requires energy. This energy can come in the form of heat, aka increase in temperature. Due to this relationship between vapor pressure and temperature, the boiling point of a liquid decreases as the atmospheric pressure decreases since there is more room above the liquid for molecules to escape into at lower pressure. The graph below illustrates this relationship using common solvents and some terpenes: Pressure vs Temperature (boiling point) Ethanol Heptane Isopropyl Alcohol B-myrcene 290.0 B-caryophyllene d-Limonene Linalool Pulegone 270.0 250.0 1,8-cineole (eucalyptol) a-pinene a-terpineol terpineol-4-ol 230.0 p-cymene 210.0 190.0 170.0 150.0 130.0 110.0 90.0 Temperature (˚C) Temperature 70.0 50.0 30.0 10 20 30 40 50 60 70 80 90 100 200 300 400 500 600 760 10.0 -10.0 -30.0 Pressure (torr) 1 Boiling Points and Vapor Pressure Background As a very general rule of thumb, the boiling point of many liquids will drop about 0.5˚C for a 10mmHg decrease in pressure when operating in the region of 760 mmHg (atmospheric pressure). -

ESSENTIALS of METEOROLOGY (7Th Ed.) GLOSSARY
ESSENTIALS OF METEOROLOGY (7th ed.) GLOSSARY Chapter 1 Aerosols Tiny suspended solid particles (dust, smoke, etc.) or liquid droplets that enter the atmosphere from either natural or human (anthropogenic) sources, such as the burning of fossil fuels. Sulfur-containing fossil fuels, such as coal, produce sulfate aerosols. Air density The ratio of the mass of a substance to the volume occupied by it. Air density is usually expressed as g/cm3 or kg/m3. Also See Density. Air pressure The pressure exerted by the mass of air above a given point, usually expressed in millibars (mb), inches of (atmospheric mercury (Hg) or in hectopascals (hPa). pressure) Atmosphere The envelope of gases that surround a planet and are held to it by the planet's gravitational attraction. The earth's atmosphere is mainly nitrogen and oxygen. Carbon dioxide (CO2) A colorless, odorless gas whose concentration is about 0.039 percent (390 ppm) in a volume of air near sea level. It is a selective absorber of infrared radiation and, consequently, it is important in the earth's atmospheric greenhouse effect. Solid CO2 is called dry ice. Climate The accumulation of daily and seasonal weather events over a long period of time. Front The transition zone between two distinct air masses. Hurricane A tropical cyclone having winds in excess of 64 knots (74 mi/hr). Ionosphere An electrified region of the upper atmosphere where fairly large concentrations of ions and free electrons exist. Lapse rate The rate at which an atmospheric variable (usually temperature) decreases with height. (See Environmental lapse rate.) Mesosphere The atmospheric layer between the stratosphere and the thermosphere. -

Ground (Subsurface) Ice Shumskii, P
NRC Publications Archive Archives des publications du CNRC Ground (subsurface) ice Shumskii, P. A.; National Research Council of Canada. Division of Building Research For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous. Publisher’s version / Version de l'éditeur: https://doi.org/10.4224/20386780 Technical Translation (National Research Council of Canada), 1964 NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=b714ab23-775f-4f1d-9a1e-ab4bc8f71c00 https://publications-cnrc.canada.ca/fra/voir/objet/?id=b714ab23-775f-4f1d-9a1e-ab4bc8f71c00 Access and use of this website and the material on it are subject to the Terms and Conditions set forth at https://nrc-publications.canada.ca/eng/copyright READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site https://publications-cnrc.canada.ca/fra/droits LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB. Questions? Contact the NRC Publications Archive team at [email protected]. If you wish to email the authors directly, please see the first page of the publication for their contact information. Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à [email protected]. -

Problem Set #10 Assigned November 8, 2013 – Due Friday, November 15, 2013 Please Show All Work for Credit
Problem Set #10 Assigned November 8, 2013 – Due Friday, November 15, 2013 Please show all work for credit To Hand in 1. 1 2. –1 A least squares fit of ln P versus 1/T gives the result Hvaporization = 25.28 kJ mol . 3. Assuming constant pressure and temperature, and that the surface area of the protein is reduced by 25% due to the hydrophobic interaction: 2 G 0.25 N A 4 r Convert to per mole, ↓determine size per molecule 4 r 3 V M N 0.73mL/ g 60000g / mol (6.02 1023) 3 2 2 A r 2.52 109 m 2 23 9 2 G 0.25 N A 4 r 0.0720N / m 0.25 6.02 10 / mol (4 ) (2.52 10 m) 865kJ / mol We think this is a reasonable approach, but the value seems high 2 4. The vapor pressure of an unknown solid is approximately given by ln(P/Torr) = 22.413 – 2035(K/T), and the vapor pressure of the liquid phase of the same substance is approximately given by ln(P/Torr) = 18.352 – 1736(K/T). a. Calculate Hvaporization and Hsublimation. b. Calculate Hfusion. c. Calculate the triple point temperature and pressure. a) Calculate Hvaporization and Hsublimation. From Equation (8.16) dPln H sublimation dT RT 2 dln P d ln P dT d ln P H T 2 sublimation 11 dT dT R dd TT For this specific case H sublimation 2035 H 16.92 103 J mol –1 R sublimation Following the same proedure as above, H vaporization 1736 H 14.43 103 J mol –1 R vaporization b. -

Novel Hydraulic Structures and Water Management in Iran: a Historical Perspective
Novel hydraulic structures and water management in Iran: A historical perspective Shahram Khora Sanizadeh Department of Water Resources Research, Water Research Institute������, Iran Summary. Iran is located in an arid, semi-arid region. Due to the unfavorable distribution of surface water, to fulfill water demands and fluctuation of yearly seasonal streams, Iranian people have tried to provide a better condition for utilization of water as a vital matter. This paper intends to acquaint the readers with some of the famous Iranian historical water monuments. Keywords. Historic – Water – Monuments – Iran – Qanat – Ab anbar – Dam. Structures hydrauliques et gestion de l’eau en Iran : une perspective historique Résumé. L’Iran est situé dans une région aride, semi-aride. La répartition défavorable des eaux de surface a conduit la population iranienne à créer de meilleures conditions d’utilisation d’une ressource aussi vitale que l’eau pour faire face à la demande et aux fluctuations des débits saisonniers annuels. Ce travail vise à faire connaître certains des monuments hydrauliques historiques parmi les plus fameux de l’Iran. Mots-clés. Historique – Eau – Monuments – Iran – Qanat – Ab anbar – Barrage. I - Introduction Iran is located in an arid, semi-arid region. Due to the unfavorable distribution of surface water, to fulfill water demands and fluctuation of yearly seasonal streams, Iranian people have tried to provide a better condition for utilization of water as a vital matter. Iran is located in the south of Asia between 44º 02´ and 63º 20´ eastern longitude and 25º 03´ to 39º 46´ northern latitude. The country covers an area of about 1.648 million km2. -

Chapter Two Properties of Ammonia
Chapter Two Properties of Ammonia Physical Properties General Anhydrous ammonia exists as either a colorless gas, colorless liquid, or white solid, depending on its pressure and temperature. In nearly all commonly encountered situations, it exists as either a liquid or a gas. The gas is less dense than air and the liquid is less dense than water at standard conditions. Ammonia gas (vapor) diffuses readily in air and the liquid is highly soluble in water with an accompanying release of heat. Ammonia exhibits classical saturation relationships whereby pressure and temperature are directly related so long as both the vapor and liquid phase are present. It does have a critical pressure and temperature. At atmospheric pressure, a closed container of ammonia vapor and liquid will be in equilibrium at a temperature of –28°F [–33°C]. It should be noted however that if liquid ammonia is spilled or released to the atmosphere at normal temperatures, the resultant pool of boiling liquid will be significantly colder than –28°F due to the law of partial pressures (the partial pressure of the ammonia vapor in the air near the liquid surface will be less than atmospheric pressure). The following table provides some of the important physical properties of ammonia. TABLE 2-1 Physical Properties of Ammonia Property Condition Value (IP) Value (SI) Molecular Weight 17.03 17.03 Color None None Physical State Room Temp Gas Gas Freezing Point P=1 atm –108°F –78°C Boiling Point P=1 atm –28.1°F –33.3°C Critical Pressure 1657 psia 11,410 kPa Critical Temp 271°F -

The Thermodynamic Properties of Nitrogen from 64 to 300* K Between
Lonal Bureau of btaudaj a.^ Lilarary, M.W. Bldg JUIV 6 1962 PB 161630 ^ecknlcciL ^^ote 129 THE THERMODYNAMIC PROPERTIES OF NITROGEN FROM 64 TO 300^ K BETWEEN 0.1 AND 200 ATMOSPHERES THOMAS R. STROBRIDGE U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the na- tional standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials,- devices, and structures; advisory services to government agen- cies on scientific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Reseeirch projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. -

Aqua Ammonia Customer Manual
CUSTOMER MANUAL – AQUA AMMONIA TABLE OF CONTENTS AQUA AMMONIA PROPERTIES. 2 AQUA AMMONIA QUALITY . 3 HAZARDOUS PROPERTIES . ... 4 AQUA AMMONIA – STRENGTH DETERMINATION. .. 4 SHIPPING CONTAINERS FOR AQUA AMMONIA . 5 STORAGE OF AQUA AMMONIA . 6 AQUA AMMONIA STORAGE TANKS . 6 BULK DELIVERIES BY CARGO TANKS . 6 MATERIALS FOR PIPING & FITTINGS . .. 7 SYSTEM DESIGN . .. 8 AQUA AMMONIA TRANSFER METHODS DRAWING # 8031 . 9 Page 1 Revision 5/98 LEGAL NOTICE: Copyright © 1998 Tanner Industries, Inc. (“TII”). This document is provided for use by customers and prospective customers of TII only. This document may not be modified, distributed, or reproduced in any form, or by any electronic or mechanical means, including the use of information storage and retrieval systems, without permission in writing from TII. All rights reserved. Unauthorized use is strictly prohibited. AQUA AMMONIA PROPERTIES Aqua ammonia, also called ammoniacal liquor, ammonia liquor or ammonia water is produced by dissolving ammonia gas in water. Its proper chemical name is Ammonium Hydroxide. The grades, or strength of Ammonium Hydroxide usually available commercially are 26° and 21° Baumé. However, Tanner Industries can produce any strength to meet our customers requirements. The Baumé reading refers to a specific gravity scale. A 26° Baumé solution is equivalent to 29.4% and 21° Baumé solution is equivalent to 19.68%, both by weight of ammonia dissolved in water. Since the Baumé reading varies with temperature, the reading is standardized at 60°F. The density of the material compared to water is 0.8974. See Aqua Ammonia Table. Aqua ammonia is corrosive to copper, cooper alloys, aluminum alloys and galvanized surfaces. -

© Cambridge University Press Cambridge
Cambridge University Press 978-0-521-80620-6 - Creep and Fracture of Ice Erland M. Schulson and Paul Duval Index More information Index 100-year wave force, 336 friction and fracture, 289, 376 60° dislocations, 17, 82, 88 indentation failure, 345 microstructure, 45, 70, 237, 255, 273 abrasion, 337 multiscale fracture and frictional accommodation processes of basal slip, 165 sliding, 386 acoustic emission, 78, 90, 108 nested envelopes, 377 across-column cleavage cracks, 278 pressure–area relationship, 349, 352 across-column confinement, 282 S2 growth texture, 246, 273 across-column cracks, 282, 306, SHEBA faults, 371 across-column loading, 275 SHEBA stress states, 377 across-column strength, 246, 249, 275 Arctic Ocean, 1, 45, 190, 361 activation energy, 71, 84, 95, 111, 118, 131 aspect ratio, 344 activation volume, 114, 182 atmospheric ice, 219, 221, 241, 243 activity of pyramidal slip systems, 158 atmospheric icing, 31 activity of slip systems, 168 atmospheric impurities, 113 adiabatic heating, 291, 348 atomic packing factor, 9 adiabatic softening, 291 audible report, 240 affine self-consistent model, 160 avalanches, 206 air bubbles, 38 air-hydrate crystals, 37 bands, 89 albedo, 363 basal activity, 162 aligned first-year sea ice, 246 basal dislocations, 77 along-column confinement, 282 basal planes, 214, along-column confining stress, 282, basal screw dislocations, 77, 87 along-column strength, 244, 275 basal shear bands, 163 ammonia dihydrate, 181 basal slip, 18, 77, 127, 228 ammonia–water system, 186 basal slip lines, 77 amorphous forms