Application Note Introduction to Relative Humidity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 5 Measures of Humidity Phases of Water
Chapter 5 Atmospheric Moisture Measures of Humidity 1. Absolute humidity 2. Specific humidity 3. Actual vapor pressure 4. Saturation vapor pressure 5. Relative humidity 6. Dew point Phases of Water Water Vapor n o su ti b ra li o n m p io d a a at ep t v s o io e en s n d it n io o n c freezing Liquid Water Ice melting 1 Coexistence of Water & Vapor • Even below the boiling point, some water molecules leave the liquid (evaporation). • Similarly, some water molecules from the air enter the liquid (condense). • The behavior happens over ice too (sublimation and condensation). Saturation • If we cap the air over the water, then more and more water molecules will enter the air until saturation is reached. • At saturation there is a balance between the number of water molecules leaving the liquid and entering it. • Saturation can occur over ice too. Hydrologic Cycle 2 Air Parcel • Enclose a volume of air in an imaginary thin elastic container, which we will call an air parcel. • It contains oxygen, nitrogen, water vapor, and other molecules in the air. 1. Absolute Humidity Mass of water vapor Absolute humidity = Volume of air The absolute humidity changes with the volume of the parcel, which can change with temperature or pressure. 2. Specific Humidity Mass of water vapor Specific humidity = Total mass of air The specific humidity does not change with parcel volume. 3 Specific Humidity vs. Latitude • The highest specific humidities are observed in the tropics and the lowest values in the polar regions. -

Investigating Applicability of Evaporative Cooling Systems for Thermal Comfort of Poultry Birds in Pakistan
applied sciences Article Investigating Applicability of Evaporative Cooling Systems for Thermal Comfort of Poultry Birds in Pakistan Hafiz M. U. Raza 1, Hadeed Ashraf 1, Khawar Shahzad 1, Muhammad Sultan 1,* , Takahiko Miyazaki 2,3, Muhammad Usman 4,* , Redmond R. Shamshiri 5 , Yuguang Zhou 6 and Riaz Ahmad 6 1 Department of Agricultural Engineering, Bahauddin Zakariya University, Bosan Road, Multan 60800, Pakistan; [email protected] (H.M.U.R.); [email protected] (H.A.); [email protected] (K.S.) 2 Faculty of Engineering Sciences, Kyushu University, Kasuga-koen 6-1, Kasuga-shi, Fukuoka 816-8580, Japan; [email protected] 3 International Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan 4 Institute for Water Resources and Water Supply, Hamburg University of Technology, Am Schwarzenberg-Campus 3, 20173 Hamburg, Germany 5 Leibniz Institute for Agricultural Engineering and Bioeconomy, Max-Eyth-Allee 100, 14469 Potsdam-Bornim, Germany; [email protected] 6 Bioenergy and Environment Science & Technology Laboratory, College of Engineering, China Agricultural University, Beijing 100083, China; [email protected] (Y.Z.); [email protected] (R.A.) * Correspondence: [email protected] (M.S.); [email protected] (M.U.); Tel.: +92-333-610-8888 (M.S.); Fax: +92-61-9210298 (M.S.) Received: 4 June 2020; Accepted: 24 June 2020; Published: 28 June 2020 Abstract: In the 21st century, the poultry sector is a vital concern for the developing economies including Pakistan. The summer conditions of the city of Multan (Pakistan) are not comfortable for poultry birds. -
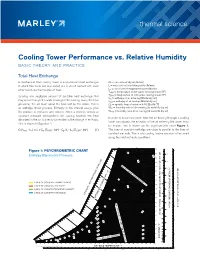
Thermal Science Cooling Tower Performance Vs. Relative Humidity
thermal science Cooling Tower Performance vs. Relative Humidity BASIC THEORY AND PRACTICE Total Heat Exchange A mechanical draft cooling tower is a specialized heat exchanger G = mass rate of dry air [lb/min] in which two fluids (air and water) are in direct contact with each L = mass rate of circulating water [lb/min] other to induce the transfer of heat. Le = mass rate of evaporated water [lb/min] THW = temperature of hot water entering tower [°F] Ignoring any negligible amount of sensible heat exchange that TCW = temperature of cold water leaving tower [°F] hin = enthalpy of air entering [Btu/lb/dry air] may occur through the walls (casing) of the cooling tower, the heat hout = enthalpy of air leaving [Btu/lb/dry air] gained by the air must equal the heat lost by the water. This is Cp = specific heat of water = 4.18 [Btu/lb-°F] an enthalpy driven process. Enthalpy is the internal energy plus Win = humidity ratio of air entering [lb water/lb dry air] the product of pressure and volume. When a process occurs at Wout = humidity ratio of air leaving [lb water/lb dry air] constant pressure (atmospheric for cooling towers), the heat In order to know how much heat the air flowing through a cooling absorbed in the air is directly correlated to the change in enthalpy. tower can absorb, the enthalpy of the air entering the tower must This is shown in Equation 1. be known. This is shown on the psychrometric chart Figure 1. G (hout - hin) = L x Cp (THW - 32°) - Cp (L - Le)(TCW - 32°) (1) The lines of constant enthalpy are close to parallel to the lines of constant wet bulb. -

Physics, Chapter 17: the Phases of Matter
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Robert Katz Publications Research Papers in Physics and Astronomy 1-1958 Physics, Chapter 17: The Phases of Matter Henry Semat City College of New York Robert Katz University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/physicskatz Part of the Physics Commons Semat, Henry and Katz, Robert, "Physics, Chapter 17: The Phases of Matter" (1958). Robert Katz Publications. 165. https://digitalcommons.unl.edu/physicskatz/165 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Robert Katz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 17 The Phases of Matter 17-1 Phases of a Substance A substance which has a definite chemical composition can exist in one or more phases, such as the vapor phase, the liquid phase, or the solid phase. When two or more such phases are in equilibrium at any given temperature and pressure, there are always surfaces of separation between the two phases. In the solid phase a pure substance generally exhibits a well-defined crystal structure in which the atoms or molecules of the substance are arranged in a repetitive lattice. Many substances are known to exist in several different solid phases at different conditions of temperature and pressure. These solid phases differ in their crystal structure. Thus ice is known to have six different solid phases, while sulphur has four different solid phases. -

Recommended Qualification Test Procedure for Solar Absorber.Pdf
Date: 2004-09-10 Editor Bo Carlsson1 IEA Solar Heating and Cooling Program Task 27 Performance of Solar Façade Components Project: Service life prediction tools for Solar Collectors Recommended qualification test procedure for solar absorber surface durability 1 Address: SP Swedish National Testing and Research Institute, P.O.Box 857, SE-50115 Borås, e-mail: [email protected] Contents Page Foreword.............................................................................................................................................................iv Introduktion .........................................................................................................................................................v 1 Scope ......................................................................................................................................................1 2 Normative references ............................................................................................................................1 3 Terms and definitions ...........................................................................................................................2 4 Requirements and classification..........................................................................................................3 5 Test methods for assessing material properties as measure of absorber performance...............4 5.1 Sampling and preparation of test specimens.....................................................................................4 -

Evaluation of the Effect of Relative Humidity of Air on the Coefficients of Critical Flow Venturi Nozzles
Evaluation of the Effect of Relative Humidity of Air on the Coefficients of Critical Flow Venturi Nozzles K. Chahine and M. Ballico National Measurement Institute, Australia P O Box 264, Lindfield, NSW 2070, Australia [email protected] Abstract atmospheric pressures. To establish stable sonic conditions in the nozzle, the down-stream end of the nozzle is usually connected to a high-capacity vacuum At NMIA, volumetric standards such as Brooks pump, with the up-stream end connected to the meter- or bell provers are used to calibrate critical flow Venturi under-test. During calibration the test-flowmeter draws nozzles or “sonic nozzles”. These nozzles, which are air from the laboratory at or near atmospheric pressures extremely stable, are used by both NMIA and Australian and temperature, and with relative humidity varying accredited laboratories to establish continuous flows for between 40% and 60%. The mass flowrate produced by the calibration of gas flow meters. For operational the nozzles is calculated based on the calibrated values of reasons, sonic nozzles are generally calibrated using dry the nozzle coefficient, the measured up-stream pressure air but later used with standard atmospheric air at various and the density calculated from the air temperature, humidity levels either drawn or blown through the meter- pressure and humidity [2]. under-test. Although the accepted theoretical calculations for determining the mass flow through a sonic nozzle At present, any effect of the relative humidity on the incorporate corrections for the resulting change in air nozzle coefficients is considered as negligible, as various density, as laboratories seek to reduce uncertainties the authors have estimated the systematic error at 0.02% for validity of this assumption warrants further examination. -

Psychrometrics Outline
Psychrometrics Outline • What is psychrometrics? • Psychrometrics in daily life and food industry • Psychrometric chart – Dry bulb temperature, wet bulb temperature, absolute humidity, relative humidity, specific volume, enthalpy – Dew point temperature • Mixing two streams of air • Heating of air and using it to dry a product 2 Psychrometrics • Psychrometrics is the study of properties of mixtures of air and water vapor • Water vapor – Superheated steam (unsaturated steam) at low pressure – Superheated steam tables are on page 817 of textbook – Properties of dry air are on page 818 of textbook – Psychrometric charts are on page 819 & 820 of textbook • What are these properties of interest and why do we need to know these properties? 3 Psychrometrics in Daily Life • Sea breeze and land breeze – When and why do we get them? • How do thunderstorms, hurricanes, and tornadoes form? • What are dew, fog, mist, and frost and when do they form? • When and why does the windshield of a car fog up? – How do you de-fog it? Is it better to blow hot air or cold air? Why? • Why do you feel dry in a heated room? – Is the moisture content of hot air lower than that of cold air? • How does a fan provide relief from sweating? • How does an air conditioner provide relief from sweating? • When does a soda can “sweat”? • When and why do we “see” our breath? • Do sailboats perform better at high or low relative humidity? Key factors: Temperature, Pressure, and Moisture Content of Air 4 Do Sailboats Perform Better at low or High RH? • Does dry air or moist air provide more thrust against the sail? • Which is denser – humid air or dry air? – Avogadro’s law: At the same temperature and pressure, the no. -

Pressure Vs Temperature (Boiling Point)
Boiling Points and Vapor Pressure Background Boiling Points and Vapor Pressure Background: Definitions Vapor Pressure: The equilibrium pressure of a vapor above its liquid; the pressure of the vapor resulting from the evaporation of a liquid above a sample of the liquid in a closed container. Boiling Point: The temperature at which the vapor pressure of a liquid is equal to the atmospheric (or applied) pressure. As the temperature of the liquid increases, the vapor pressure will increase, as seen below: https://www.chem.purdue.edu/gchelp/liquids/vpress.html Vapor pressure is interpreted in terms of molecules of liquid converting to the gaseous phase and escaping into the empty space above the liquid. In order for the molecules to escape, the intermolecular forces (Van der Waals, dipole- dipole, and hydrogen bonding) have to be overcome, which requires energy. This energy can come in the form of heat, aka increase in temperature. Due to this relationship between vapor pressure and temperature, the boiling point of a liquid decreases as the atmospheric pressure decreases since there is more room above the liquid for molecules to escape into at lower pressure. The graph below illustrates this relationship using common solvents and some terpenes: Pressure vs Temperature (boiling point) Ethanol Heptane Isopropyl Alcohol B-myrcene 290.0 B-caryophyllene d-Limonene Linalool Pulegone 270.0 250.0 1,8-cineole (eucalyptol) a-pinene a-terpineol terpineol-4-ol 230.0 p-cymene 210.0 190.0 170.0 150.0 130.0 110.0 90.0 Temperature (˚C) Temperature 70.0 50.0 30.0 10 20 30 40 50 60 70 80 90 100 200 300 400 500 600 760 10.0 -10.0 -30.0 Pressure (torr) 1 Boiling Points and Vapor Pressure Background As a very general rule of thumb, the boiling point of many liquids will drop about 0.5˚C for a 10mmHg decrease in pressure when operating in the region of 760 mmHg (atmospheric pressure). -

Model 1830, 1850 & 1850W Dehumidifier Owner's Manual
Model 1830, 1850 & 1850W Dehumidifier Owner’s Manual PLEASE LEAVE THIS MANUAL WITH THE HOMEOWNER Installed by: _________________________________ Installer Phone: _______________________ Date Installed: _______________ ON/OFF button Up/Down Dehumidifer Control Outlet used to turn buttons used to dehumidifier on change humidity and off setting MODE button used for optional ventilation feature Inlet Filter Access Drain Power Door Switch 90-1874 WHOLE HOME Dehumidification The Aprilaire® Dehumidifier controls the humidity level in your entire home. A powerful blower inside the dehumidifier draws air into the cabinet, filters the air and removes moisture, then discharges the dry air into the HVAC system or dedicated area of the home. Inside the cabinet, a sealed refrigeration system removes moisture by moving the air through a series of tubes and fins that are kept colder than the dew point of the incoming air. The dew point is the temperature at which moisture in the air will condense, much like what occurs on the outside of a cold glass on a hot summer day. The condensed moisture drips into the dehumidifier drain pan to a drain tube routed to the nearest floor drain or condensate pump. After the moisture is removed, the air moves through a second coil where it is reheated before being sent back into the home. The air leaving the dehumidifier will be warmer and drier than the air entering the dehumidifier. SETTING THE DESIRED HUMIDITY LEVEL The dehumidifier on-board control will display the humidity setting when not running, and ENERGY SavinGS TIPS displays the measured humidity when running. Energy Savings Tip #1: Adjust the humidity setting to be as high as is comfortable to reduce dehumidifier run time. -

ESSENTIALS of METEOROLOGY (7Th Ed.) GLOSSARY
ESSENTIALS OF METEOROLOGY (7th ed.) GLOSSARY Chapter 1 Aerosols Tiny suspended solid particles (dust, smoke, etc.) or liquid droplets that enter the atmosphere from either natural or human (anthropogenic) sources, such as the burning of fossil fuels. Sulfur-containing fossil fuels, such as coal, produce sulfate aerosols. Air density The ratio of the mass of a substance to the volume occupied by it. Air density is usually expressed as g/cm3 or kg/m3. Also See Density. Air pressure The pressure exerted by the mass of air above a given point, usually expressed in millibars (mb), inches of (atmospheric mercury (Hg) or in hectopascals (hPa). pressure) Atmosphere The envelope of gases that surround a planet and are held to it by the planet's gravitational attraction. The earth's atmosphere is mainly nitrogen and oxygen. Carbon dioxide (CO2) A colorless, odorless gas whose concentration is about 0.039 percent (390 ppm) in a volume of air near sea level. It is a selective absorber of infrared radiation and, consequently, it is important in the earth's atmospheric greenhouse effect. Solid CO2 is called dry ice. Climate The accumulation of daily and seasonal weather events over a long period of time. Front The transition zone between two distinct air masses. Hurricane A tropical cyclone having winds in excess of 64 knots (74 mi/hr). Ionosphere An electrified region of the upper atmosphere where fairly large concentrations of ions and free electrons exist. Lapse rate The rate at which an atmospheric variable (usually temperature) decreases with height. (See Environmental lapse rate.) Mesosphere The atmospheric layer between the stratosphere and the thermosphere. -

Factors Affecting Indoor Air Quality
Factors Affecting Indoor Air Quality The indoor environment in any building the categories that follow. The examples is a result of the interaction between the given for each category are not intended to site, climate, building system (original be a complete list. 2 design and later modifications in the Sources Outside Building structure and mechanical systems), con- struction techniques, contaminant sources Contaminated outdoor air (building materials and furnishings, n pollen, dust, fungal spores moisture, processes and activities within the n industrial pollutants building, and outdoor sources), and n general vehicle exhaust building occupants. Emissions from nearby sources The following four elements are involved n exhaust from vehicles on nearby roads Four elements— in the development of indoor air quality or in parking lots, or garages sources, the HVAC n loading docks problems: system, pollutant n odors from dumpsters Source: there is a source of contamination pathways, and or discomfort indoors, outdoors, or within n re-entrained (drawn back into the occupants—are the mechanical systems of the building. building) exhaust from the building itself or from neighboring buildings involved in the HVAC: the HVAC system is not able to n unsanitary debris near the outdoor air development of IAQ control existing air contaminants and ensure intake thermal comfort (temperature and humidity problems. conditions that are comfortable for most Soil gas occupants). n radon n leakage from underground fuel tanks Pathways: one or more pollutant pathways n contaminants from previous uses of the connect the pollutant source to the occu- site (e.g., landfills) pants and a driving force exists to move n pesticides pollutants along the pathway(s). -

Indoor Air Quality in Commercial and Institutional Buildings
Indoor Air Quality in Commercial and Institutional Buildings OSHA 3430-04 2011 Occupational Safety and Health Act of 1970 “To assure safe and healthful working conditions for working men and women; by authorizing enforcement of the standards developed under the Act; by assisting and encouraging the States in their efforts to assure safe and healthful working conditions; by providing for research, information, education, and training in the field of occupational safety and health.” This publication provides a general overview of a particular standards-related topic. This publication does not alter or determine compliance responsibili- ties which are set forth in OSHA standards, and the Occupational Safety and Health Act of 1970. More- over, because interpretations and enforcement poli- cy may change over time, for additional guidance on OSHA compliance requirements, the reader should consult current administrative interpretations and decisions by the Occupational Safety and Health Review Commission and the courts. Material contained in this publication is in the public domain and may be reproduced, fully or partially, without permission. Source credit is requested but not required. This information will be made available to sensory- impaired individuals upon request. Voice phone: (202) 693-1999; teletypewriter (TTY) number: 1-877- 889-5627. Indoor Air Quality in Commercial and Institutional Buildings Occupational Safety and Health Administration U.S. Department of Labor OSHA 3430-04 2011 The guidance is advisory in nature and informational in content. It is not a standard or regulation, and it neither creates new legal obligations nor alters existing obligations created by OSHA standards or the Occupational Safety and Health Act.