Advices for Studying Organic Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers
Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers • Ethers (R–O–R’): – Organic derivatives of water, having two organic groups bonded to the same oxygen atom © 2016 Cengage Learning 2 NAMES AND PROPERTIES OF ETHERS 3 Nomenclature: Common Names • Simple ethers are named by identifying two organic substituents and adding the word ether – Name the groups in alphabetical order – Symmetrical: Use dialkyl or just alkyl © 2016 Cengage Learning 4 Nomenclature: IUPAC Names • The more complex alkyl group is the parent name • The group with the oxygen becomes an alkoxy group © 2016 Cengage Learning 5 Nomenclature: Cyclic Ethers (Heterocycles) • Heterocyclic: Oxygen is part of the ring. O • Epoxides (oxiranes) H2C CH2 O • Oxetanes • Furans (Oxolanes) O O • Pyrans (Oxanes) O O O • Dioxanes O © 2013 Pearson Education, Inc. 6 Epoxide Nomenclature • Name the starting alkene and add “oxide” © 2013 Pearson Education, Inc. 7 Epoxide Nomenclature • The oxygen can be treated as a substituent (epoxy) on the compound • Use numbers to specify position • Oxygen is 1, the carbons are 2 and 3 • Substituents are named in alphabetical order © 2013 Pearson Education, Inc. 8 Properties of Ethers • Possess nearly the same geometry as water – Oxygen atom is sp3-hybridized – Bond angles of R–O–R bonds are approximately tetrahedral • Polar C—O bonds © 2013 Pearson Education, Inc. 9 Properties of Ethers: Hydrogen Bond • Hydrogen bond is a attractive interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom • Ethers cannot hydrogen bond with other ether molecules, so they have a lower boiling point than alcohols • Ether molecules can hydrogen bond with water and alcohol molecules • They are hydrogen bond acceptors © 2013 Pearson Education, Inc. -

Advices for Studying Organic Chemistry
Table of Contents Partial table of contents: Carbon Compounds and Chemical Bonds. Representative Carbon Compounds. An Introduction to Organic Reactions: Acids and Bases. Alkanes and Cycloalkanes: Conformations of Molecules. Stereochemistry: Chiral Molecules. Alkenes and Alkynes I: Properties and Synthesis. Alkenes and Alkynes II: Addition Reactions. Radical Reactions. Alcohols and Ethers. Conjugated Unsaturated Systems. Aromatic Compounds. Reactions of Aromatic Compounds. Aldehydes and Ketones I: Nucleophilic Additions to the Carbonyl Group. Aldehydes and Ketones II: Aldol Reactions. Carboxylic Acids and Their Derivatives: Nucleophilic Substitution at the Acyl Carbon. Amines. Carbohydrates. Lipids. Answers to Selected Problems. Glossary. Index. Solomons/Advices ADVICES FOR STUDYING ORGANIC CHEMISTRY 1. Keep up with your studying day to day –– never let yourself get behind, or better yet, be a little ahead of your instructor. Organic chemistry is a course in which one idea almost always builds on another that has gone before. 2. Study materials in small units, and be sure that you understand each new section before you go on to the next. Because of the cumulative nature of organic chemistry, your studying will be much more effective if you take each new idea as it comes and try to understand it completely before you move onto the nest concept. 3. Work all of the in-chapter and assigned problems. 4. Write when you study. Write the reactions, mechanisms, structures, and so on, over and over again. You need to know the material so thoroughly that you can explain it to someone else. This level of understanding comes to most of us (those of us without photographic memories) through writing. -
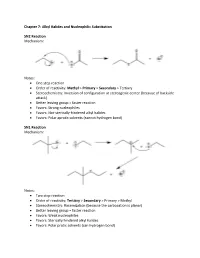
Alkyl Halides and Nucleophilic Substitution SN2 Reaction
Chapter 7: Alkyl Halides and Nucleophilic Substitution SN2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Methyl > Primary > Secondary > Tertiary • Stereochemistry: Inversion of configuration at stereogenic center (because of backside attack) • Better leaving group = faster reaction • Favors: Strong nucleophiles • Favors: Not-sterically-hindered alkyl halides • Favors: Polar aprotic solvents (cannot hydrogen bond) SN1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary > Methyl • Stereochemistry: Racemization (because the carbocation is planar) • Better leaving group = faster reaction • Favors: Weak nucleophiles • Favors: Sterically hindered alkyl halides • Favors: Polar protic solvents (can hydrogen bond) Important Trends Chapter 8: Alkyl Halides and Elimination Reactions E2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: antiperiplanar arrangement of H and X • Better leaving group = faster reaction • Favors: Polar aprotic solvents, strong bases • Products follow Zaitsev rule (more substituted alkene is the major product) E1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: Trigonal planar carbocation intermediate • Better leaving group = faster reaction • Favors: Polar protic solvents, weak bases • Products follow Zaitsev rule Chapter 9: Alcohols, Ethers, and Epoxides Preparation of Alcohols Mechanism: Notes: • SN2 mechanism -

Reactions of Alkenes Since Bonds Are Stronger Than Bonds, Double Bonds Tend to React to Convert the Double Bond Into Bonds
Reactions of Alkenes Since bonds are stronger than bonds, double bonds tend to react to convert the double bond into bonds This is an addition reaction. (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic. Electrophilic Addition The bond is localized above and below the C-C bond. The electrons are relatively far away from the nuclei and are therefore loosely bound. An electrophile will attract those electrons, and can pull them away to form a new bond. This leaves one carbon with only 3 bonds and a +ve charge (carbocation). The double bond acts as a nucleophile (attacks the electrophile). Ch08 Reacns of Alkenes (landscape) Page 1 In most cases, the cation produced will react with another nucleophile to produce the final overall electrophilic addition product. Electrophilic addition is probably the most common reaction of alkenes. Consider the electrophilic addition of H-Br to but-2-ene: The alkene abstracts a proton from the HBr, and a carbocation and bromide ion are generated. The bromide ion quickly attacks the cationic center and yields the final product. In the final product, H-Br has been added across the double bond. Ch08 Reacns of Alkenes (landscape) Page 2 Orientation of Addition Consider the addition of H-Br to 2-methylbut-2-ene: There are two possible products arising from the two different ways of adding H-Br across the double bond. But only one is observed. The observed product is the one resulting from the more stable carbocation intermediate. Tertiary carbocations are more stable than secondary. -

Reaction-Of-Alkenes.Pdf
8 Reactions of Alkenes Goals for Chapter 8 1 Explain why electrophilic additions are among the most common reactions of alkenes. femoral component 2 Predict the products of the reactions of alkenes, including the orientation of the reaction (regio- polyethylene bearing chemistry) and the stereochemistry. tibial plate 3 Propose mechanisms to explain the observed products of alkene reactions. 4 Use retrosynthetic analysis to solve multistep synthesis problems with alkenes as reagents, intermediates, or products. ◀ Polyethylene provides a self-lubricating surface for movement of the metal parts of an artificial knee replacement. The highly cross-linked polyethylene used in these implants is fabricated to be exceptionally tough: It wears only about 0.1 mm per year. Polyethylene is compatible with the human body, and in most cases, it does not cause a foreign body reaction even after years of constant movement in the joint. The alkene double bond is a gateway functional group. Alkene reactions lead to many other functional groups that lay the foundation for the rest of your study of organic chemistry. You can convert alkenes to alkyl halides, epoxides, alcohols, aldehydes, ketones, carboxylic acids, and other functional groups. The reactions of alkenes arise from the reactivity of their carbon–carbon double bonds. Organic chemists enjoy the challenge of taking a simple carbon–carbon double bond and manipulating it in all possible ways to produce other compounds, often mimicking biological reactions that occur in cells. This chapter covers the most common alkene reactions, including their mechanisms, reactivity, orientation, and stereochemistry. Most reactions of alkenes involve addition of atoms or groups across the double bond, with one atom or group adding to each end. -

Reactions of Alkenes
Reactions of Alkenes Alkenes generally react in an addition mechanism (addition – two new species add to a molecule and none leave) R X Y X Y H R R R H Have already observed using a H+ electrophile (HBr or H+/H2O) that a carbocation intermediate is generated which directs the regiochemistry Whenever a free carbocation intermediate is generated there will not be a stereopreference due to the nucleophile being able to react on either lobe of the carbocation (already observed this with SN1 and E1 reactions) Br Br H+ H H3C Br CH2CH3 Obtain racemic mixture of this regioisomer Reactions of Alkenes There are three questions to ask for any addition reaction R X Y X Y H R R R H 1) What is being added? (what is the electrophile?) 2) What is the regiochemistry? (do the reagents add with the X group to the left or right?) 3) What is the stereochemistry? (do both the X and Y groups add to the same side of the double bond or opposite sides?) All of these questions can be answered if the intermediate structure is known Reactions of Alkenes Dihalogen compounds can also react as electrophiles in reactions with alkenes Possible partial bond structures + !+ Br Br ! Br Br Br !+ or Br !+ More stable partial positive charge Experimentally it is known, however, that rearrangements do nor occur with Br2 addition -therefore free carbocations must not be present The large size and polarizability of the halogen can stabilize the unstable carbocation With an unsymmetrical alkene, however, both bonds to the bromine need not be equivalent Called a “Bromonium” ion -

P880-183197 Ii I 11\11111 Ii Iii Ii 11111 1111111 1111111
EPA-560/ll-80-005 P880-183197 II I 11\11111 II III II 11111 1111111 1111111 INVESTIGATION OF SELECTED POTENTIAL ENVIRONMENTAL CONTAMINANTS: EPOXIDES Dennis A. Bogyo Sheldon S. Lande William M. Meylan Philip H. Howard Joseph Santodonato March 1980 FINAL REPORT Contract No. 68-01-3920 SRC No. L1342-05 Project Officer - Frank J. Letkiewicz Prepared for: Office of Toxic Substances U.S. Environmental Protection Agency Washington, D.C. 20460 Document is available to the public through the National Technical Information Service, Springfield, Virginia 22151 REPRODUCED BY CE U S DEPARTMENT OF COMMER ., NATIONAL TECHNICAL INFORMATION SERVICE SPRINGFIELD, VA 22161 TECHNICAL REPORT DATA (Please read illWtictiollS on the reverse before completing) I REPORT NO. 2 3. RECIPIENT'S ACCESSIO~NO. EPA-560/ll-80-005 1 . ~~~~ llf03:;n~? -l. TITLE AND SUBTITLE S. REPORT DATE Investigation of Selected Potential March 1980 Environmental Contaminants: Epoxides 6. PERFORMING ORGANIZATION CODE 7 AuTHORiS) 8. PERFORMING ORGANIZATION REPORT NO Dennis A. Bogyo, Sheldon S. Lande, William M. Meylan, TR 80;...535 Philip H. Howard, Joseph Santodonato 9. PERFORMING ORGANIZATION NAME AND ADDRESS 10. PROGRAM ELEMENT NO. Center for Chemical Hazard Assessment Syracuse Research Corporation 11. CONTRACT/GRANT NO. Merrill Lane EPA 68-01-3920 Syracuse, New York 13210 12. SPONSORING AGENCY NAME AND ADDRESS 13. TYPE OF REPORT AND PERIOD COVERED Final Technical Report - Office of· Toxic Substances 14. SPONSORING AGENCY CODE U.S. Environmental Protection Agency Washington, D.C. 20460 15. SUPPLEMENTARY NOTES 16. ABSTRACT This report reviews the potential environmental and health hazards associated with the commercial use of selected epoxide compounds. -

Organic Chemistry I
Organic Chemistry I Andrew Rosen April 11, 2013 Contents 1 The Basics Bonding and Molecular Structure 6 1.1 We Are Stardust . .6 1.2 Atomic Structure . .6 1.3 The Structural Theory of Organic Chemistry . .6 1.4 Chemical Bonds: The Octet Rule; How to Write Lewis Structures; Exceptions to the Octet Rule..................................................6 1.5 Formal Charges . .6 1.6 Resonance Theory . .6 1.6.1 Determination of Stability for Resonance Structures . .7 1.7 Quantum Mechanics and Atomic Structure . .7 1.8 Atomic Orbitals and Electron Conguration . .7 1.8.1 Orbitals . .7 1.8.2 Congurations . .8 1.8.3 Rules of Conguration . .8 1.9 Molecular Orbitals . .8 1.10 The Structure of Methane and Ethane: sp3 Hybridization . .8 1.11 The Structure of Ethene: sp2 Hybridization . .9 1.12 The Structure of Ethyne (Acetylene): sp Hybridization . .9 1.13 Techniques for Drawing Resonance Structures Systematically . 10 1.14 How to Interpret and Write Structural Formulas; Applications of Basic Principles . 10 1.15 Molecular Geometry: The Valence Shell Electron Repulsion (VSEPR) Model . 11 2 Families of Carbon Compounds 12 2.1 Hydrocarbons: Representative Alkanes, Alkenes, Alkynes, and Aromatic Compounds . 12 2.2 Polar Covalent Bonds . 12 2.3 Polar and Nonpolar Molecules . 13 2.4 Functional Groups . 13 2.5 Alkyl Halides or Haloalkanes . 13 2.6 Alcohols and Ethers . 13 2.7 Amines . 14 2.8 Aldehydes and Ketones . 14 2.9 Carboxylic Acids, Esters, Amides, and Nitriles . 14 2.10 Summary of Important Families of Organic Compounds . 15 1 2.11 Physical Properties of Molecular Structure . -

Ethers and Epoxides; Thiols and Sulfides
Ethers and Epoxides; Thiols and Sulfides Introduction • Ethers are compounds with two organic groups (alkyl, aryl, or vinyl) bonded to the same oxygen atom, R–O–R’, in a ring or in an open chain Thiols (R–S–H) • Thiols (R–S–H) and sulfides (R–S–R’) are sulfur analogs of alcohols and ethers, respectively • Sulfur replaces oxygen Naming Ethers • Ethers are named according to IUPAC rules: – Simple ethers with no other functional groups are named by identifying the two organic substituents and adding the word ether – If other functional groups are present, the ether part is considered an alkoxy substituent – Simple ethers with no other functional groups are named by identifying the two organic substituents and adding the word ether – If other functional groups are present, the ether part is considered an alkoxy substituent Practice Problem: Name the following ethers according to IUPAC rules Diisopropyl ether Cyclopentyl propyl ether 1-Methoxycyclohexene p-Bromoanisole or Ethyl isobutyl ether 4-Bromo-1- Allyl vinyl ether methoxybenzene Structure and Properties of Ethers • The geometry around the O atom of an ether (ROR) is similar to that of water (HOH) – R-O-R has an ~ tetrahedral bond angle (112° in dimethyl ether) – The O atom is sp3-hybridized • The oxygen atom gives ethers a slight dipole moment Ethers have higher boiling points than alkanes with similar MW Synthesis of Ethers • Ethers can be synthesized by: i. Acid-catalyzed dehydration of alcohols ii. Williamson ether synthesis iii. Alkoxymercuration of alkenes Acid-catalyzed dehydration of alcohols • Symmetrical ethers can be prepared by acid-catalyzed dehydration of primary alcohols (SN2) – Example: Diethyl ether is prepared industrially by sulfuric acid–catalyzed dehydration of ethanol – Acid-catalyzed dehydration of secondary and tertiary alcohols yield alkenes (E1) The Williamson Ether Synthesis • Symmetrical and unsymmetrical ethers can be prepared via the Williamson ether synthesis. -

Workbook for Organic Chemistry Supplemental Problems and Solutions
This page intentionally left blank This page intentionally left blank WORKBOOK FOR ORGANIC CHEMISTRY SUPPLEMENTAL PROBLEMS AND SOLUTIONS Jerry A. Jenkins Otterbein College W.H. Freeman and Company New York © 2010 by W.H. Freeman and Company All rights reserved. Printed in the United States of America ISBN-13: 978-1-4292-4758-0 ISBN-10: 1-4292-4758-4 First printing W.H. Freeman and Company 41 Madison Avenue New York, NY 10010 Houndmills, Basingstoke RG21 6XS England www.whfreeman.com/chemistry TABLE OF CONTENTS PREFACE v About the author vi | Acknowledgments vi | Selected concepts/reactions locator vii TIPS viii | Common abbreviations ix CHAPTER 1 THE BASICS 1 1.1 Hybridization, formulas, physical properties 1 | 1.2 Acids and bases 4 | 1.3 Resonance 7 CHAPTER 2 ALKANES 11 2.1 General 11 | 2.2 Nomenclature 12 | 2.3 Conformational analysis, acyclic 13 CHAPTER 3 CYCLOALKANES 15 3.1 General 15 | 3.2 Nomenclature 16 | 3.3 Conformational analysis, cyclic 18 CHAPTER 4 REACTION BASICS 21 CHAPTER 5 ALKENES AND CARBOCATIONS 27 5.1 General 27 | 5.2 Reactions 30 | 5.3 Syntheses 36 | 5.4 Mechanisms 39 CHAPTER 6 ALKYNES 49 6.1 Reactions 49 | 6.2 Syntheses 50 | 6.3 Mechanisms 53 CHAPTER 7 STEREOCHEMISTRY 55 7.1 General 55 | 7.2 Reactions and stereochemistry 61 CHAPTER 8 ALKYL HALIDES AND RADICALS 65 8.1 Reactions 65 | 8.2 Syntheses 66 | 8.3 Mechanisms 67 CHAPTER 9 SN1, SN2, E1, AND E2 REACTIONS 69 9.1 General 69 | 9.2 Reactions 71 | 9.3 Syntheses 76 | 9.4 Mechanisms 78 CHAPTER 10 NMR 87 CHAPTER 11 CONJUGATED SYSTEMS 93 11.1 Reactions 93 | 11.2 Syntheses 96 | 11.3 Mechanisms 98 CHAPTER 12 AROMATICS 103 12.1 General 103 | 12. -

Chapter 10 Lecture Outline
Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai’i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright © 2017 McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education. 1 Electrophilic Addition of Alcohols • Alcohols add to alkenes, forming ethers by the same mechanism. • For example, addition of CH3OH to 2-methylpropene, forms tert-butyl methyl ether (MTBE), a high octane fuel additive. End class 11/10/17Friday 2 3 Halogenation—Electrophilic Addition of Halogen • Halogenation is the addition of X2 (X = Cl or Br) to an alkene to form a vicinal dihalide. (anti addition) 4 Halogenation Mechanism 5 Halohydrin Formation • Treatment of an alkene with a halogen X2 and H2O forms a halohydrin by addition of the groups of X and OH to the double bond. 6 Mechanism of Halohydrin Formation • Even though X¯ is formed in step [1] of the mechanism, its concentration is small compared to H2O (often the solvent), so H2O and not X¯ is the nucleophile. 7 Anti Stereochemistry in Halohydrin Formation • With unsymmetrical alkenes, the preferred product has the electrophile X+ bonded to the less substituted carbon, and the nucleophile (H2O) bonded to the more substituted carbon. 8 Regiochemistry of Halohydrin Formation • As in the acid catalyzed ring opening of epoxides, nucleophilic attack occurs at the more substituted carbon end of the bridged halonium ion because that carbon is better able to accommodate the partial positive charge in the transition state. 9 Summary of Halohydrin Formation 10 Halohydrin Use in Synthesis • Halohydrins have been used in the synthesis of many naturally occurring compounds.