Amino Acids and Proteins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A-PDF Split DEMO : Purchase from to Remove the Watermark
A-PDF Split DEMO : Purchase from www.A-PDF.com to remove the watermark Figure 7.5 (a) Transition state for E2H-syn elimination. (b) Transition state for E2H-anti elimination. (c) Transition state for E,C elimination. tion state similar to that shown in Figure 7.5~is expected, since base attacks the molecule backside to the leaving group but frontside to the /3 hydrogen. Let us now turn to the experimental results to see if these predictions are borne out in fact. It has long been known that E2H reactions normally give preferentially anti elimination. For example, reaction of meso-stilbene dibromide with potassium ethoxide gives cis-bromostilbene (Reaction 7.39), whereas reaction of the D,L-dibromide gives the trans product (Reaction 7.40).lo2 A multitude of other examples exist-see, for example, note 64 (p. 355) and note 82 (p. 362). E2H reactions do, however, give syn elimination when: (1) an H-X di- hedral angle of 0" is achievable but one of 180" is not or, put another way, H and X can become syn-periplanar but not trans-periplanar ; (2) a syn hydrogen is much more reactive than the anti ones; (3) syn elimination is favored for steric reasons; and (4) an anionic base that remains coordinated with its cation, that, in turn, is coordinated with the leaving group, is used as catalyst. The very great importance of category 4 has only begun to be fully realized in the early 1970s. lo2 P. Pfeiffer, 2. Physik. Chem. Leibrig, 48, 40 (1904). 1,2-Elimination Reactions 371 An example of category 1 is found in the observation by Brown and Liu that eliminations from the rigid ring system 44, induced by the sodium salt of 2- cyclohexylcyclohexanol in triglyme, produces norborene (98 percent) but no 2-de~teronorbornene.~~~The dihedral angle between D and tosylate is O", but Crown ether present: No Yes that between H and tosylate is 120". -
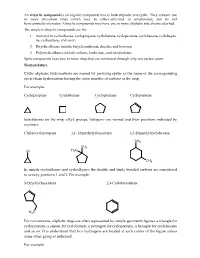
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

The Smallest Quantum Vortex
“The 2D Symmetry of Nature” T.M. Lach April 11, 2005 The structures of nature and the strong nuclear force The Proton the smallest Quantum Vortex Over the years many new discoveries have taught us that nature works its miracles with very simple structures. Going back 100 years we see this simplicity in structures like the Benzene molecule, Polycyclic Aromatic Hydrocarbons (PAH), the Hydrogen atom, DNA, snowflakes and crystal lattices. (1) In recent years many new discoveries, like the discovery of Quarks, have reinforced this belief. Ninety years ago, Niels Bohr’s model of the structure of the hydrogen atom (March 6 th 1913) revolutionized science since it brought together a few simple principles that allowed us to understand the nature of why atoms have quantized energy levels. Thus began the new era of Quantum Theory and Quantum Mechanics. Many before Bohr had assumed that the electrons were circling the nucleus. (2) Nicholson earlier had assumed that the angular momentum of the electron was quantized, for 30 years others had tried to explain the Balmer series of the hydrogen spectrum, but Bohr tied it all together into a coherent picture of the hydrogen atom. Bohr had to assume that the circling electrons did not lose energy to radiation and thereby spiral into the nucleus, a big assumption at the time. Ten years later in 1923 DeBroglie proposed the wave nature of particles, which explained why the circling electrons could maintain stable quantized orbits and so began the quantum nature of the universe, with a little help from Plank, Einstein, Shrodinger, Sommerfeld and many others. -

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis
New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Author: Jason Joseph Marineau Persistent link: http://hdl.handle.net/2345/1741 This work is posted on eScholarship@BC, Boston College University Libraries. Boston College Electronic Thesis or Dissertation, 2010 Copyright is held by the author, with all rights reserved, unless otherwise noted. Boston College The Graduate School of Arts and Sciences Department of Chemistry NEW APPLICATIONS OF CYCLOBUTADIENE CYCLOADDITIONS: DIVERSITY AND TARGET ORIENTED SYNTHESIS a dissertation by JASON JOSEPH MARINEAU submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2010 © Copyright by JASON JOSEPH MARINEAU 2010 New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Jason J. Marineau Thesis Advisor: Professor Marc. L. Snapper Abstract Cyclobutadiene cycloadditions provide rapid access to rigid polycyclic systems with high strain energy and unusual molecular geometries. Further functionalization of these systems allows entry into unexplored chemical space. A tricarbonylcyclobutadiene iron complex on solid support enables exploration of these cycloadditions in a parallel format amenable to diversity oriented synthesis. Modeling of the cycloaddition transition states with density functional calculations provides a theoretical basis for analysis of the regioselectivity observed in generation of these substituted bicyclo[2.2.0]hexene derivatives. The high strain energy accessible in cyclobutadiene cycloadducts and their derivatives renders them useful synthons for access to medium-ring natural products through ring expansion. Torilin, a guaiane sesquiterpene isolated from extracts of the fruits of Torilis japonica, exhibits a range of biological activities including testosterone 5 -reductase inhibition, hKv1.5 channel blocking, hepatoprotective, anti-inflammatory and anti-cancer effects. -

Conformational Analysis of Molecular Chains Using Nano-Kinematics
Conformational analysis of molecular chains using Nano-Kinematics y z Dinesh Mano cha Yunshan Zhu William Wright Computer Science Department UniversityofNorth Carolina Chap el Hill, NC 27599-317 5 fmano cha,zhu,[email protected] Fax: 919962-179 9 Abstract: We present algorithms for 3-D manipulation and conformational analysis of molecular chains, when b ond length, b ond angles and related dihedral angles remain xed. These algorithms are useful for lo cal deformations of linear molecules, exact ring closure in cyclic molecules and molecular emb edding for short chains. Other p ossible applications include structure prediction, protein folding, conformation energy analysis and 3D molecular matching and do cking. The algo- rithms are applicable to all serial molecular chains and make no asssumptions ab out their geometry. We make use of results on direct and inverse kinematics from rob otics and mechanics literature and show the corresp ondence b etween kinematics and conformational analysis of molecules. In particular, we p ose these problems algebraically and compute all the solutions making use of the structure of these equations and matrix computations. The algorithms have b een implemented and p erform well in practice. In particular, they take tens of milliseconds on currentworkstations for lo cal deformations and chain closures on molecular chains consisting of six or fewer rotatable dihedral angles. Categories: 3D Protein Mo deling, 3D Molecular Matching and Do cking, Multiple alignmentof genetic sequences Supp orted in part -

Chapter 1 Tropone and Tropolone
School of Molecular and Life Sciences New Routes to Troponoid Natural Products Jason Matthew Wells This thesis is presented for the Degree of Doctor of Philosophy of Curtin University November 2018 Declaration To the best of my knowledge and belief this thesis contains no material previously pub- lished by any other person except where due acknowledgement has been made. This thesis contains no material which has been accepted for the award of any other degree or diploma in any other university. Signature: Date: i Abstract Malaria is an infectious disease found in humans and other animals, it is caused by a single-cell parasite of the Plasmodium genus with many different substrains. Of these, P. falciparum is the most deadly to humans causing the majority of deaths. Although research into the area of antimalarial compounds is wide spread, few have been devel- oped with new structural features. Cordytropolone 37 is a natural product isolated in 2001 from the insect pathogenic fungus Cordyceps sp. BCC 1681 and has been shown to have antimalarial activity against P. falciparum. It has a structure unrelated to antimalarial com- pounds currently used in therapy. It does not contain a peroxide bridge as with artemisinin 25 or quinoline rings as with chloroquine 22. This unique structure indicates that it could possibly interact with the malaria parasite in a fashion unlike current treatments. In order for cordytropolone to be further developed as a potential treatment, it must first be synthe- sised in a laboratory environment. This study attempts to develop the first total synthesis of cordytropolone. H HO O N O O N O N H H H O O Cl HO O 22 25 37 Figure 0.0.1: Cordytropolone 37 has a unique structure compared to the current common malaria treatments The first method investigated towards the total synthesis of cordytropolone involved an intramolecular Buchner ring expansion. -

Here Science-Based Cost- Effective Pathways Forward?
Sponsors Institute of Atomic and Molecular Sciences, Academia Sinica, Taiwan PIRE-ECCI Program, UC Santa Barbara, USA Max-Planck-Gesellschaft, Germany National Science Council, Taiwan Organizing committee Dr. Kuei-Hsien Chen (IAMS, Academia Sinica, Taiwan) Dr. Susannah Scott (University of California - Santa Barbara, USA) Dr. Alec Wodtke (University of Göttingen & Max-Planck-Gesellschaft, Germany) Table of Contents General Information .............................................................................................. 1 Program for Sustainable Energy Workshop ....................................................... 3 I01 Dr. Alec M. Wodtke Beam-surface Scattering as a Probe of Chemical Reaction Dynamics at Interfaces ........................................................................................................... 7 I02 Dr. Kopin Liu Imaging the steric effects in polyatomic reactions ............................................. 9 I03 Dr. Chi-Kung Ni Energy Transfer of Highly Vibrationally Excited Molecules and Supercollisions ................................................................................................. 11 I04 Dr. Eckart Hasselbrink Energy Conversion from Catalytic Reactions to Hot Electrons in Thin Metal Heterostructures ............................................................................................... 13 I05 Dr. Jim Jr-Min Lin ClOOCl and Ozone Hole — A Catalytic Cycle that We Don’t Like ............... 15 I06 Dr. Trevor W. Hayton Nitric Oxide Reduction Mediated by a Nickel Complex -

A 1 Case-PR/ }*Rciofft.;Is Report
.A 1 case-PR/ }*rciofft.;is Report (a) This eruption site on Mauna Loa Volcano was the main source of the voluminous lavas that flowed two- thirds of the distance to the town of Hilo (20 km). In the interior of the lava fountains, the white-orange color indicates maximum temperatures of about 1120°C; deeper orange in both the fountains and flows reflects decreasing temperatures (<1100°C) at edges and the surface. (b) High winds swept the exposed ridges, and the filter cannister was changed in the shelter of a p^hoehoc (lava) ridge to protect the sample from gas contamination. (c) Because of the high temperatures and acid gases, special clothing and equipment was necessary to protect the eyes. nose, lungs, and skin. Safety features included military flight suits of nonflammable fabric, fuil-face respirators that are equipped with dual acidic gas filters (purple attachments), hard hats, heavy, thick-soled boots, and protective gloves. We used portable radios to keep in touch with the Hawaii Volcano Observatory, where the area's seismic activity was monitored continuously. (d) Spatter activity in the Pu'u O Vent during the January 1984 eruption of Kilauea Volcano. Magma visible in the circular conduit oscillated in a piston-like fashion; spatter was ejected to heights of 1 to 10 m. During this activity, we sampled gases continuously for 5 hours at the west edge. Cover photo: This aerial view of Kilauea Volcano was taken in April 1984 during overflights to collect gas samples from the plume. The bluish portion of the gas plume contained a far higher density of fine-grained scoria (ash). -

Fine Tuning the Hydrophilic–Hydrophobic Balance in Inositols Through Annulation: an Analysis of the Hydrogen-Bonded Architectures of ‘Annulated Inositols’
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Publications of the IAS Fellows PAPER www.rsc.org/crystengcomm | CrystEngComm Fine tuning the hydrophilic–hydrophobic balance in inositols through annulation: An analysis of the hydrogen-bonded architectures of ‘annulated inositols’ Goverdhan Mehta* and Saikat Sen Received 13th September 2005, Accepted 2nd November 2005 First published as an Advance Article on the web 15th November 2005 DOI: 10.1039/b512911g The crystal structures of conformationally locked, bicyclic cycloalkane-annulated variants of myo- and chiro-inositols have been analysed, in order to understand their mode of expression of the axial rich conformations and amphiphilicity in the solid state. Thus, while cyclohexa- annulated myo- and chiro-inositols exhibit a head-to-head bilayer molecular assembly, consisting of dimeric or octameric columnar architectures, cyclopenta-annulated chiro-inositol shows no such aggregation of the hydrophilic and hydrophobic faces, rather preferring to pack in a manner akin to a hydrophilic inositol. The differences in the two packing modes can be attributed to the size of the hydrocarbon ring, fine-tuning the hydrophilic–hydrophobic balance in the annulated inositols. An analysis of the non-polar molecular surface area (a measure of the intermolecular hydrophobic van der Waals interactions) for all the annulated inositols under study further vindicates the conclusion. Introduction alicyclic ring, could serve as a handle to fine tune the hydrophilic–hydrophobic balance in the otherwise polar Cyclohexane-1,2,3,4,5,6-hexol, in all its nine stereoisomeric inositols. This could in principle serve as a means of forms, constitutes a group of biologically important entities, modulating the cell membrane permeability and receptor 1 commonly known as inositols. -

Mass Spectrometry : Apparatus
Mass – Spectrometry Mass spectrometry : Apparatus Mass spectrometry :Aparatus MASS Spectrum of acetophenone Fragment Ions Molecular ion (mass ion) MASS Spectrum of Benzamide Mass spectrometry: Processing steps of the sample 1. Ionization of molecules 2. Fragmentation of ionized molecules 3. Acceleration of ions 4. Analysys of the ions Resolution of mass spectrometer 푀 푀푛 10001 푅 = = = = 10000 Δ푀 푀푛−푀푚 10001−10000 M m Mn H ℎ ∗ 100 ≤ 10 % 퐻 Ion sources • 1. Electron ionization (EI) (Electron Impact) • 2. Chemical Ionization (CI) • 3. Fast Atom Bombardment (FAB) • 4. Laser Desorption (LD) • 5. Matrix-Assisted Laser Desorption Ionization (MALDI) • 6. ElectroSpray ionization (ESI) Electron Ionization (EI) – Ionization Chamber Electron Ionization (EI) What is going on physically? Electron Ionization - Energy of electrons Each electron is associated to a wave whose wavelength λ is given by ℎ λ = 푚 υ where m is its mass, v its velocity and h Planck’s constant. Wavelength is 2.7Å for a kinetic energy of 20 eV and 1.4 Å for 70 eV. Number of ions produced as a function of the electron energy. Advantages of EI 1. Reproducible method 2. High Ionization Efficiency 3. All vaporized molecules can be ionized (non polar and insoluble) 4. Molecular structural information (fragmentation) Disadvantages of EI 1. Only +ve ions are formed 2. Sample has to be volatile 3. Limits to 600Da or less MW 4. Extensive fragmentation Chemical Ionization (CI) 1. Sample is injected in atmosfere of gas (methane, izobutane, ammonia). 2. Gas is ionised by EI method. 3. During the collisions of methane ions with molecules of sample, energy is ransfered, as well as protons is transfered. -

Reference Guide for Medicinal & Organic
Reference Guide for Medicinal and Organic Chemistry Krisman - Questions and Answers REFERENCE GUIDE FOR MEDICINAL & ORGANIC CHEMISTRY QUESTIONS & ANSWERS 2009-2010 Manan H. Shroff www.pharmacyexam.com 1 Reference Guide for Medicinal and Organic Chemistry Krisman - Questions and Answers REFERENCE GUIDE FOR MEDICINAL & ORGANIC CHEMISTRY QUESTIONS & ANSWERS 2009-2010 www.pharmacyexam.com 2 Reference Guide for Medicinal and Organic Chemistry Krisman - Questions and Answers This reference guide is not intended as a substitute for the advice of a physician. Students or readers must consult their physician about any existing problem. Do not use any information in this reference guide for any kind of self-treatment. Do not administer any dose of mentioned drugs in this reference guide without consulting your physician. This is only a review guide for preparation for the Foreign Pharmacy Licensing board exam. The author of this reference guide is not responsible for any kind of misinterpreted, incorrect or misleading information or any typographical errors in this guide. Any doubtful or questionable answers should be checked in other available reference sources. All rights reserved. No part of this guide may be reproduced or transmitted in any form or by any means, electroni- cally photocopied, recorded or otherwise , without prior written permission of the publisher. RXEXAM is a registered trademark of Pharmacy Exam of Krishna Publication Inc. Any unauthorized use of this trademark will be considered a violation of law. NAPLEX and FPGEE are federally registered trademarks owned by the National Associa- tion of Boards of Pharmacy (NABP) and this reference guide is in no way authorized or spon- sored by NABP.