A-PDF Split DEMO : Purchase from www.A-PDF.com to remove the watermark
A-PDF Split DEMO : Purchase from to Remove the Watermark
Total Page:16
File Type:pdf, Size:1020Kb
Recommended publications
-
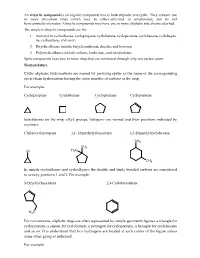
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis
New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Author: Jason Joseph Marineau Persistent link: http://hdl.handle.net/2345/1741 This work is posted on eScholarship@BC, Boston College University Libraries. Boston College Electronic Thesis or Dissertation, 2010 Copyright is held by the author, with all rights reserved, unless otherwise noted. Boston College The Graduate School of Arts and Sciences Department of Chemistry NEW APPLICATIONS OF CYCLOBUTADIENE CYCLOADDITIONS: DIVERSITY AND TARGET ORIENTED SYNTHESIS a dissertation by JASON JOSEPH MARINEAU submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2010 © Copyright by JASON JOSEPH MARINEAU 2010 New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Jason J. Marineau Thesis Advisor: Professor Marc. L. Snapper Abstract Cyclobutadiene cycloadditions provide rapid access to rigid polycyclic systems with high strain energy and unusual molecular geometries. Further functionalization of these systems allows entry into unexplored chemical space. A tricarbonylcyclobutadiene iron complex on solid support enables exploration of these cycloadditions in a parallel format amenable to diversity oriented synthesis. Modeling of the cycloaddition transition states with density functional calculations provides a theoretical basis for analysis of the regioselectivity observed in generation of these substituted bicyclo[2.2.0]hexene derivatives. The high strain energy accessible in cyclobutadiene cycloadducts and their derivatives renders them useful synthons for access to medium-ring natural products through ring expansion. Torilin, a guaiane sesquiterpene isolated from extracts of the fruits of Torilis japonica, exhibits a range of biological activities including testosterone 5 -reductase inhibition, hKv1.5 channel blocking, hepatoprotective, anti-inflammatory and anti-cancer effects. -

Here Science-Based Cost- Effective Pathways Forward?
Sponsors Institute of Atomic and Molecular Sciences, Academia Sinica, Taiwan PIRE-ECCI Program, UC Santa Barbara, USA Max-Planck-Gesellschaft, Germany National Science Council, Taiwan Organizing committee Dr. Kuei-Hsien Chen (IAMS, Academia Sinica, Taiwan) Dr. Susannah Scott (University of California - Santa Barbara, USA) Dr. Alec Wodtke (University of Göttingen & Max-Planck-Gesellschaft, Germany) Table of Contents General Information .............................................................................................. 1 Program for Sustainable Energy Workshop ....................................................... 3 I01 Dr. Alec M. Wodtke Beam-surface Scattering as a Probe of Chemical Reaction Dynamics at Interfaces ........................................................................................................... 7 I02 Dr. Kopin Liu Imaging the steric effects in polyatomic reactions ............................................. 9 I03 Dr. Chi-Kung Ni Energy Transfer of Highly Vibrationally Excited Molecules and Supercollisions ................................................................................................. 11 I04 Dr. Eckart Hasselbrink Energy Conversion from Catalytic Reactions to Hot Electrons in Thin Metal Heterostructures ............................................................................................... 13 I05 Dr. Jim Jr-Min Lin ClOOCl and Ozone Hole — A Catalytic Cycle that We Don’t Like ............... 15 I06 Dr. Trevor W. Hayton Nitric Oxide Reduction Mediated by a Nickel Complex -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/75818 Please be advised that this information was generated on 2018-07-08 and may be subject to change. SULFATION VIA SULFITE AND SULFATE DIESTERS AND SYNTHESIS OF BIOLOGICALLY RELEVANT ORGANOSULFATES Een wetenschappelijke proeve op het gebied van de Natuurwetenschappen, Wiskunde en Informatica Proefschrift ter verkrijging van de graad van doctor aan de Radboud Universiteit Nijmegen op gezag van de rector magnificus prof. mr. S. C. J. J. Kortmann, volgens besluit van het college van decanen in het openbaar te verdedigen op vrijdag 9 oktober 2009 om 13:00 uur precies door Martijn Huibers geboren op 21 november 1978 te Arnhem Promotor: Prof. dr. Floris P. J. T. Rutjes Copromotor: Dr. Floris L. van Delft Manuscriptcommissie: Prof. dr. ir. Jan C. M. van Hest Prof. dr. Binne Zwanenburg Dr. Martin Ostendorf (Schering‐Plough) Paranimfen: Laurens Mellaard Eline van Roest Het in dit proefschrift beschreven onderzoek is uitgevoerd in het kader van het project “Use of sulfatases in the production of sulfatated carbohydrates and steroids”. Dit project is onderdeel van het Integration of Biosynthesis and Organic Synthesis (IBOS) programma, wat deel uitmaakt van het Advanced Chemical Technologies for Sustainability (ACTS) platform van de Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO). Cofinancierders zijn Schering‐Plough en Syncom. ISBN/EAN: 978‐94‐90122‐51‐5 Drukkerij: Gildeprint Drukkerijen, Enschede Omslagfoto: Martijn Huibers i.s.m. Maarten van Roest Omslagontwerp: Martijn Huibers en Gildeprint Drukkerijen Vermelding van een citaat (onderaan kernpagina's 1 tot en met 112) betekent niet per se dat de auteur van dit proefschrift zich daarbij aansluit, maar dat de uitspraak interessant is, prikkelend is, en/of betrekking heeft op de wetenschap in het algemeen of dit promotieonderzoek in het bijzonder. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0183866A1 Pohl Et Al
US 2006O183866A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0183866A1 Pohl et al. (43) Pub. Date: Aug. 17, 2006 (54) BLOCKED MERCAPTOSILANE COUPLING (60) Provisional application No. 60/056,566, filed on Aug. AGENTS FOR FILLED RUBBERS 21, 1997. (76) Inventors: Eric R. Pohl, Mount Kisco, NY (US); Publication Classification Richard W. Cruse, Yorktown Heights, NY (US); Keith J. Weller, North (51) Int. Cl. Greenbush, NY (US); Robert J. CSF I32/00 (2006.01) Pickwell, Tonawanda, NY (US) (52) U.S. Cl. ...................... 525/326.1; 524/146; 524/167; 524/168; 524/173; 524/262: Correspondence Address: 524/265; 524/267: 524/302: DILWORTH & BARRESE, LLP 524/306; 524/307; 524/308; 333 EARLE OVINGTON BLVD. 524/309; 524/311:524/392: UNIONDALE, NY 11553 (US) 524/393; 524/401 (21) Appl. No.: 11/398,125 (57) ABSTRACT (22) Filed: Apr. 5, 2006 Blocked mercaptosilanes are provided in which the blocking Related U.S. Application Data group contains an unsaturated heteroatom or carbon chemi cally bound directly to sulfur via a single bond. This (60) Continuation of application No. 09/986,512, filed on blocking group optionally may be substituted with one or Nov. 9, 2001, which is a division of application No. more carboxylate ester or carboxylic acid functional groups. 09/284.841, filed on Apr. 21, 1999, now Pat. No. These silanes are used in manufacture of inorganic filled 6,414,061, filed as 371 of international application rubbers, with the silanes deblocked by a deblocking agent. No. PCT/US98/17391, filed on Aug. -

The X1 Method for Accurate and Efficient Prediction of Heats of Formation
THE JOURNAL OF CHEMICAL PHYSICS 127, 214105 ͑2007͒ The X1 method for accurate and efficient prediction of heats of formation ͒ Jianming Wu and Xin Xua State Key Laboratory of Physical Chemistry of Solid Surfaces and Center for Theoretical Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China ͑Received 31 July 2007; accepted 25 September 2007; published online 5 December 2007͒ We propose the X1 method which combines the density functional theory method with a neural network ͑NN͒ correction for an accurate yet efficient prediction of heats of formation. It calculates the final energy by using B3LYP/6-311+G͑3df,2p͒ at the B3LYP/6-311+G͑d,p͒ optimized geometry to obtain the B3LYP standard heats of formation at 298 K with the unscaled zero-point energy and thermal corrections at the latter basis set. The NN parameters cover 15 elements of H, Li, Be, B, C, N, O, F, Na, Mg, Al, Si, P, S, and Cl. The performance of X1 is close to the Gn theories, giving a mean absolute deviation of 1.43 kcal/mol for the G3/99 set of 223 molecules up to 10 nonhydrogen atoms and 1.48 kcal/mol for the X1/07 set of 393 molecules up to 32 nonhydrogen atoms. © 2007 American Institute of Physics. ͓DOI: 10.1063/1.2800018͔ I. INTRODUCTION the B3LYP method to calculate heats of formation of neutral organic molecules. Their results are promising. Calibrated ͑⌬ ͒ The accurate prediction of heats of formation Hf is with their own compilation of 180 organic molecules for the one of the central topics in computational chemistry. -

Oxidized Sulfur Functionalized Polymers Via Admet Polymerization
OXIDIZED SULFUR FUNCTIONALIZED POLYMERS VIA ADMET POLYMERIZATION By TAYLOR WILLIAM GAINES A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2015 © 2015 Taylor William Gaines To Mom and Steve ACKNOWLEDGMENTS There are so many who have been contributors to my success and I would like thank all of them. This doctorate involved not only the four years in graduate school, but everything in life up until the degree’s competition. I have always tried to remember life’s little lessons. I cannot mention everyone who deserves thanks; otherwise, this section would comprise the entire document. But I believe those mentioned below have had the most profound effect on my life and especially my education. I must start by first thanking my grandparents, Iris and Tony Pritchard, who were essential to my early upbringing in western North Carolina. These two were hardworking, small town, blue-collar, and family oriented. My grandfather was in the automotive repair industry, and my grandmother worked 47 years for the same furniture company. They grew up in the depression, knew the value of hard work, family, and how to make a little money go a long way. I can honestly say they never met a stranger, and they were kind to everyone. I am most appreciative of the values these two instilled in me, including dedication, the importance of family, and the value of hard work. They loved us unconditionally and spoiled my brother and me, probably too much at times. -

Development of a Protecting Group for Sulfate Esters
Development of a Protecting Group for Sulfate Esters. Andrew D. Proud. FIIJ The University of Edinburgh. II,9J C) Abstract: This work describes the development of a protecting group for sulfate monoesters in carbohydrates. After initial trial studies, the trifluoroethyl ester was selected as a suitable protecting group. The ester was formed from the sulfate by treatment with trifluorodiazoethane. This protection method is shown to be compatible with other common protecting groups used in carbohydrate chemistry. The protecting group is proven to be stable to the manipulations of many other protecting groups. The utility of monosaccharides incorporating protected sulfate esters in synthesis has been examined. It has been proven that they are useful and versatile building blocks in the synthesis of more complex structures. Selective cleavage of the trifluoroethyl ester was achieved with potassium tert-butoxide. The mechanism for this deprotection reaction has been studied and this investigation has led to the advancement of alternative deprotection methods. I declare that, this Thesis has been composed by m,yself and that the work is my own. Andrew David Proud. Acknowledgements: I would like to thank my supervisor Prof. Sabine Flitsch for her help and support throughout this project. I also wish to thank Dr. Jeremy Prodger, of GlaxoWelicome and Prof. Nick Turner of The University of Edinburgh for their many suggestions and useful advice. I wish to acknowledge the EPSRC and GlaxoWelicome for financial support. I wish to thank all the people that run the NMR and X-ray crystallography services at Edinburgh University but especially John Miller and Simon Parsons. -

View PDF Version
ChemComm View Article Online COMMUNICATION View Journal | View Issue Sulfation made simple: a strategy for synthesising sulfated molecules† Cite this: Chem. Commun., 2019, 55,4319 Daniel M. Gill,a Louise Maleb and Alan M. Jones *a Received 5th February 2019, Accepted 12th March 2019 DOI: 10.1039/c9cc01057b rsc.li/chemcomm The study of organosulfates is a burgeoning area in biology, yet there are significant challenges with their synthesis. We report the development of a tributylsulfoammonium betaine as a high yielding route to organosulfates. The optimised reaction conditions were Creative Commons Attribution 3.0 Unported Licence. interrogated with a diverse range of alcohols, including natural products and amino acids. Organosulfates play a variety of important roles in biology, from xenobiotic metabolism to the downstream signalling of steroidal Fig. 1 Examples of drug molecules containing organosulfates: heparin, s s sulfates in disease states.1 Sulfate groups on glycosaminoglycans sotradecol and avibactam ; a chemical tool compound (C3)anda metabolite of paracetamol. (GAGs) such as heparin, heparan sulfate and chondroitin sulfate, facilitate molecular interactions and protein ligand binding at the cellular surface,2 an area of interest in drug discovery.3 A variety of methods to prepare organosulfates are shown in This article is licensed under a Heparin (an anticoagulant),4 avibactams (a b-lactamase Chart 1. inhibitor),5 sotradecol (a treatment for varicose veins),6 the The preparation of organosulfate esters include a microwave 7 sulfate metabolite of paracetamol (an analgesic), and the assisted approach to the sulfation of alcohols, using Me3NSO3 and 8 15,16 Open Access Article. Published on 12 March 2019. -

I a Study of the Basicities of Some Aryl Methyl Sulfoxides Ii a Study of the Hydrolysis of Arenesulfinamides in Basic Aqueous Ethanol
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 1968 I A STUDY OF THE BASICITIES OF SOME ARYL METHYL SULFOXIDES II A STUDY OF THE HYDROLYSIS OF ARENESULFINAMIDES IN BASIC AQUEOUS ETHANOL JOSEPH BRIAN BIASOTTI Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation BIASOTTI, JOSEPH BRIAN, "I A STUDY OF THE BASICITIES OF SOME ARYL METHYL SULFOXIDES II A STUDY OF THE HYDROLYSIS OF ARENESULFINAMIDES IN BASIC AQUEOUS ETHANOL" (1968). Doctoral Dissertations. 871. https://scholars.unh.edu/dissertation/871 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received ^ ^ BIASOTTI, Joseph Brian, 1942- I. A STUDY OF THE BASICITIES OF SOME ARYL METHYL SULFOXIDES H. A STUDY OF THE HYDROLYSIS OF ARENESULFINAMIDES IN BASIC AQUEOUS ETHANOL. University of New Hampshire, Ph.D., 1968 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan I. A STUDY OF THE BASICITIES OF SOME ARYL METHYL SULFOXIDES . A STUDY OF THE HYDROLYSIS OF ARENESULFINAMIDES IN BASIC AQUEOUS ETHANOL j T b r i a n b i a s o t t i B. S., Boston College, 1964 A THESIS Submitted to the University of New Hampshire In Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Graduate School Department of Chemistry June, 1968 ABSTRACT I. -

(12) STANDARD PATENT (11) Application No. AU 2008255138 B2 (19) AUSTRALIAN PATENT OFFICE
(12) STANDARD PATENT (11) Application No. AU 2008255138 B2 (19) AUSTRALIAN PATENT OFFICE (54) Title Therapeutic treatments for blood cell deficiencies (51) International Patent Classification(s) A61K 31/00 (2006.01) A61K 31/739 (2006.01) A61K 31/122 (2006.01) A61K 33/00 (2006.01) A61K 31/17 (2006.01) A61K 33/14 (2006.01) A61K 31/19 (2006.01) A61K 35/74 (2006.01) A61K 31/198 (2006.01) A61K 38/00 (2006.01) A61K 31/407 (2006.01) A61P 7/00 (2006.01) A61K 31/429 (2006.01) A61P 13/12 (2006.01) A61K 31/56 (2006.01) C07J 1/00 (2006.01) A61K 31/565 (2006.01) C07J 17/00 (2006.01) A61K 31/568 (2006.01) C07J 73/00 (2006.01) (21) Application No: 2008255138 (22) Date of Filing: 2008.12.04 (43) Publication Date: 2009.01.08 (43) Publication Journal Date: 2009.01.08 (44) Accepted Journal Date: 2011.02.24 (62) Divisional of: 2005237117 (71) Applicant(s) Harbor Biosciences, Inc. (72) Inventor(s) Prendergast, Patrick T.;Frincke, James Martin;Reading, Christopher L. (74) Agent / Attorney Griffith Hack, Level 3 509 St Kilda Road, Melbourne, VIC, 3004 (56) Related Art NL 99772 ABSTRACT The present invention provides a method to prevent a blood cell deficiency such as neutropenia or thrombocytopenia by administering to a subject an effective amount of a certain steroid. N:\Melboume\Cases\Patent\44000-44999\P44912.AU.2\Specis\P44912 AU-2 Specafication 2008-12-4.doc 4112108 AUSTRALIA Patents Act 1990 COMPLETE SPECIFICATION Standard Patent Applicant (s) HOLLIS-EDEN PHARMACEUTICALS, INC.