Cucurbiturils: from Synthesis to High-Affinity Binding and Catalysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A-PDF Split DEMO : Purchase from to Remove the Watermark
A-PDF Split DEMO : Purchase from www.A-PDF.com to remove the watermark Figure 7.5 (a) Transition state for E2H-syn elimination. (b) Transition state for E2H-anti elimination. (c) Transition state for E,C elimination. tion state similar to that shown in Figure 7.5~is expected, since base attacks the molecule backside to the leaving group but frontside to the /3 hydrogen. Let us now turn to the experimental results to see if these predictions are borne out in fact. It has long been known that E2H reactions normally give preferentially anti elimination. For example, reaction of meso-stilbene dibromide with potassium ethoxide gives cis-bromostilbene (Reaction 7.39), whereas reaction of the D,L-dibromide gives the trans product (Reaction 7.40).lo2 A multitude of other examples exist-see, for example, note 64 (p. 355) and note 82 (p. 362). E2H reactions do, however, give syn elimination when: (1) an H-X di- hedral angle of 0" is achievable but one of 180" is not or, put another way, H and X can become syn-periplanar but not trans-periplanar ; (2) a syn hydrogen is much more reactive than the anti ones; (3) syn elimination is favored for steric reasons; and (4) an anionic base that remains coordinated with its cation, that, in turn, is coordinated with the leaving group, is used as catalyst. The very great importance of category 4 has only begun to be fully realized in the early 1970s. lo2 P. Pfeiffer, 2. Physik. Chem. Leibrig, 48, 40 (1904). 1,2-Elimination Reactions 371 An example of category 1 is found in the observation by Brown and Liu that eliminations from the rigid ring system 44, induced by the sodium salt of 2- cyclohexylcyclohexanol in triglyme, produces norborene (98 percent) but no 2-de~teronorbornene.~~~The dihedral angle between D and tosylate is O", but Crown ether present: No Yes that between H and tosylate is 120". -
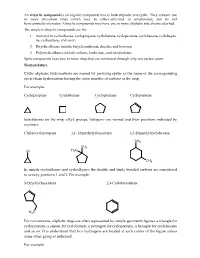
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

Cucurbituril Assembly and Uses Thereof Cucurbiturilanordnung Und Seine Verwendungen Ensemble De Cucurbituril Et Ses Utilisations
(19) TZZ_Z__T (11) EP 1 668 015 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: C07D 487/22 (2006.01) C07D 519/00 (2006.01) of the grant of the patent: G01N 33/00 (2006.01) C08G 73/00 (2006.01) 21.08.2013 Bulletin 2013/34 (86) International application number: (21) Application number: 04770467.1 PCT/IL2004/000796 (22) Date of filing: 05.09.2004 (87) International publication number: WO 2005/023816 (17.03.2005 Gazette 2005/11) (54) CUCURBITURIL ASSEMBLY AND USES THEREOF CUCURBITURILANORDNUNG UND SEINE VERWENDUNGEN ENSEMBLE DE CUCURBITURIL ET SES UTILISATIONS (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR WO-A-00/68232 WO-A-03/004500 HU IE IT LI LU MC NL PL PT RO SE SI SK TR WO-A-03/055888 WO-A-03/055888 (30) Priority: 04.09.2003 US 499735 P • JON, SANG YONG ET AL: "Facile Synthesis of 13.01.2004 US 535829 P Cucurbit[n]uril Derivatives via Direct Functionalization: Expanding Utilization of (43) Date of publication of application: Cucurbit[n]uril" JOURNAL OF THE AMERICAN 14.06.2006 Bulletin 2006/24 CHEMICAL SOCIETY , 125(34), 10186-10187 CODEN: JACSAT; ISSN: 0002-7863, 8 June 2003 (73) Proprietor: TECHNION RESEARCH AND (2003-06-08), XP002316247 DEVELOPMENT FOUNDATION, LTD. • JON, SANG YONG ET AL: "Facile Synthesis of Haifa 32000 (IL) Cucurbit[n]uril Derivatives via Direct Functionalization: Expanding Utilization of (72) Inventor: KEINAN, Ehud Cucurbit[n]uril" JOURNAL OF THE AMERICAN 23 840 Timrat (IL) CHEMICAL SOCIETY , 125(34), 10186-10187 CODEN: JACSAT; ISSN: 0002-7863, 2003, (74) Representative: Dennemeyer & Associates S.A. -

New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis
New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Author: Jason Joseph Marineau Persistent link: http://hdl.handle.net/2345/1741 This work is posted on eScholarship@BC, Boston College University Libraries. Boston College Electronic Thesis or Dissertation, 2010 Copyright is held by the author, with all rights reserved, unless otherwise noted. Boston College The Graduate School of Arts and Sciences Department of Chemistry NEW APPLICATIONS OF CYCLOBUTADIENE CYCLOADDITIONS: DIVERSITY AND TARGET ORIENTED SYNTHESIS a dissertation by JASON JOSEPH MARINEAU submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2010 © Copyright by JASON JOSEPH MARINEAU 2010 New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Jason J. Marineau Thesis Advisor: Professor Marc. L. Snapper Abstract Cyclobutadiene cycloadditions provide rapid access to rigid polycyclic systems with high strain energy and unusual molecular geometries. Further functionalization of these systems allows entry into unexplored chemical space. A tricarbonylcyclobutadiene iron complex on solid support enables exploration of these cycloadditions in a parallel format amenable to diversity oriented synthesis. Modeling of the cycloaddition transition states with density functional calculations provides a theoretical basis for analysis of the regioselectivity observed in generation of these substituted bicyclo[2.2.0]hexene derivatives. The high strain energy accessible in cyclobutadiene cycloadducts and their derivatives renders them useful synthons for access to medium-ring natural products through ring expansion. Torilin, a guaiane sesquiterpene isolated from extracts of the fruits of Torilis japonica, exhibits a range of biological activities including testosterone 5 -reductase inhibition, hKv1.5 channel blocking, hepatoprotective, anti-inflammatory and anti-cancer effects. -

Study on the Complexation of Macromolecule Cucurbituril with Metals and Acetamide
International Journal of Chemistry and Applications. ISSN 0974-3111 Volume 4, Number 3 (2012), pp. 219-226 © International Research Publication House http://www.irphouse.com Study on the Complexation of Macromolecule Cucurbituril with Metals and Acetamide T. Jaba Priya, Jasmine Rebecca and R. Wilfred Sugumar Department of Chemistry, Madras Christian College (Autonomous), Tambaram, Chennai-600059, India. E-mail: [email protected]; [email protected] Abstract Cucurbituril is a remarkably robust macro cyclic host molecule that has a rigid cavity capable of binding small guests under a variety of conditions. In the present study, macromolecule cucurbit[6]uril (CB[6]) and its complexes with metal ions lithium, cerium were synthesized. Mixed ligand complexes containing CB[6] and acetamide with central metal ions such as lithium were also prepared. The UV – visible spectrum of the complex of lithium with CB[6] shows a peak at 225nm. The mixed ligand complex of lithium with CB[6] and acetamide shows a sharp peak at 220nm which indicates the presence of amide functional group. The peak at 274nm indicates the presence of – NO2 group. UV-visible spectrum of the complex of cerium with CB[6] shows a peak at 213.17nm, which indicates the presence of – CONH2 group. The IR spectrum of Cucurbituril shows the following peaks 3436.96 cm-1 , 2931.16 to 2372.15 cm-1 and a sharp peak at 1726.39 cm-1 these frequencies indicate the presence of CO - N ,C-H and C=O stretching respectively. The IR spectra of the complex of Lithium with CB[6] and Cerium with CB[6] show significant variations especially around 3000 cm-1 and between 1700 to 1200 cm-1 indicating prevalence of enhanced hydrogen bonding. -

Here Science-Based Cost- Effective Pathways Forward?
Sponsors Institute of Atomic and Molecular Sciences, Academia Sinica, Taiwan PIRE-ECCI Program, UC Santa Barbara, USA Max-Planck-Gesellschaft, Germany National Science Council, Taiwan Organizing committee Dr. Kuei-Hsien Chen (IAMS, Academia Sinica, Taiwan) Dr. Susannah Scott (University of California - Santa Barbara, USA) Dr. Alec Wodtke (University of Göttingen & Max-Planck-Gesellschaft, Germany) Table of Contents General Information .............................................................................................. 1 Program for Sustainable Energy Workshop ....................................................... 3 I01 Dr. Alec M. Wodtke Beam-surface Scattering as a Probe of Chemical Reaction Dynamics at Interfaces ........................................................................................................... 7 I02 Dr. Kopin Liu Imaging the steric effects in polyatomic reactions ............................................. 9 I03 Dr. Chi-Kung Ni Energy Transfer of Highly Vibrationally Excited Molecules and Supercollisions ................................................................................................. 11 I04 Dr. Eckart Hasselbrink Energy Conversion from Catalytic Reactions to Hot Electrons in Thin Metal Heterostructures ............................................................................................... 13 I05 Dr. Jim Jr-Min Lin ClOOCl and Ozone Hole — A Catalytic Cycle that We Don’t Like ............... 15 I06 Dr. Trevor W. Hayton Nitric Oxide Reduction Mediated by a Nickel Complex -
![Cucurbit[7]Uril Host-Viologen Guest Complexes](https://docslib.b-cdn.net/cover/3644/cucurbit-7-uril-host-viologen-guest-complexes-423644.webp)
Cucurbit[7]Uril Host-Viologen Guest Complexes
CUCURBIT[7]URIL HOST-VIOLOGEN GUEST COMPLEXES: ELECTROCHROMIC AND PHOTOCHEMICAL PROPERTIES by MARINA FREITAG A dissertation submitted to the Graduate School – Newark Rutgers, The State University of New Jersey in partial fulfillment of requirements for the degree of Doctor of Philosophy Graduate Program in Chemistry Written under the direction of Professor Elena Galoppini and approved by ________________________ ________________________ ________________________ ________________________ Newark, New Jersey October, 2011 ABSTRACT OF THE DISSERTATION Abstract Cucurbituril[7] Host - Viologen Guest Complexes: Electrochromic and Photochemical Properties By MARINA FREITAG Dissertation Director: Professor Elena Galoppini In this thesis, we demonstrated that a molecular host, cucurbit[7]uril, provides an alternative method of adsorbing molecules on semiconductors and shields the guest from the hetereogenous interface. These novel hybrid systems exhibited photophysical and electrochemical properties that differ from the properties of layers obtained by directly attaching the chromophore to the semiconductor through binding groups. This thesis describes the host-guest chemistry between cucurbit[7]uril (CB[7]) and various series of viologen guests. Methylviologen (1,1'-dimethyl-4,4'-bipyridinium dichloride, MV2+), 1-methyl-1'-p-tolyl-4,4'-bipyridinium dichloride (MTV2+), and 1,1'-di- p-tolyl-(4,4'-bipyridine)-1,1'-diium dichloride (DTV2+) were encapsulated in the macrocyclic host cucurbit[7]uril, CB[7]. The complexes MV2+@CB[7] and MTV2+@CB[7] were physisorbed to the surface of 1 TiO2 nanoparticle films. The complexation into CB[7] was monitored by H NMR. TiO2 films functionalized with the complexes were studied by FT-IR-ATR and UV-Vis ii absorption. The electrochemical and spectroelectrochemical properties of MV2+@CB[7] and MTV2+@CB[7] were studied in solution and in electrochromic windows (ECDs), where the complexes were bound to TiO2 films cast on FTO. -

Formation of Functionalized Supramolecular Metallo-Organic Oligomers with Cucurbituril a Thesis Presented to the Faculty Of
Formation of Functionalized Supramolecular Metallo-organic Oligomers with Cucurbituril A thesis presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Master of Science Ian M. Del Valle December 2015 © 2015 Ian M. Del Valle. All Rights Reserved. 2 This thesis titled Formation of Functionalized Supramolecular Metallo-organic Oligomers with Cucurbituril by IAN M. DEL VALLE has been approved for the Department of Chemistry and Biochemistry and the College of Arts and Sciences by Eric Masson Associate Professor of Chemistry and Biochemistry Robert Frank Dean, College of Arts and Sciences 3 Abstract DEL VALLE, IAN M., M.S., December 2015, Chemistry Formation of Functionalized Supramolecular Metallo-organic Oligomers with Cucurbituril Director of Thesis: Eric Masson The goal of this project is to functionalize supramolecular oligomer chains with amino acids and nucleic acids in order to observe interactions with proteins and DNA. Chiral substituents are also desirable to induce helicality in the oligomer much like DNA. We explored different pathways to afford these oligomers. The first project involves forming metallo-organic oligomers using non-covalent bonds and then functionalizing them. We synthesize ligands and use alkyne-azide cycloadditions to functionalize them. These ligands can then be coordinated to various transition metals. The aromatic regions of these oligomers can then self-assemble into tube-like chains with the participation of cucurbit[8]uril. Second, we explore an alternate pathway to form functionalized chains. This second set of chains coupled amines with carboxylic acid groups attached to the ligands. This project hopes to avoid solubility problems experienced with the first project. -

Synthesis and Characterization of Supramolecular Cucurbituril, Molybdenum 2,2'-Bipyridyl Complex
International Journal of Scientific & Engineering Research Volume 8, Issue 10, October-2017 140 ISSN 2229-5518 SYNTHESIS AND CHARACTERIZATION OF SUPRAMOLECULAR CUCURBITURIL, MOLYBDENUM 2,2’-BIPYRIDYL COMPLEX Ms. Anitha. V1 , Dr. Nandagopal. J2, Ms. Ramanachiar.R3, Ms. Sasikala.S4, Ms. Ariyamala .R5 Department of chemistry, Velammal Engineering college, Chennai, Tamilnadu. Abstract Supra molecular Cucurbituril, Molybdenum2,2’-bipyridyl complex has been synthesized by precipitation followed by slow evaporation techniques. Changes in the physical properties of the ligand and the complex were characterized by melting point and solubility test . The absorption spectrum of the Cucurbituril, Molybdenum2,2’-bipyridyl complex were studied by decreasing and increasing concentrations by using UV – Visible spectrophotometer and IR spectrophotometer. The interaction between the metal and ligands were studied by UV- visible spectral analysis. The functional groups and modes of vibration were identified by IR spectral analysis. Key words: Cucurbituril, evaporation, precipitation, absorption, ligand, spectrophotometer. INTRODUCTION complex of Cucurbit[6]uril which incorporates the p-Xylene diammonium cation into the macrocyclic A Supramolecular assembly or “supramolecule” is a cavity. Researchers have shown that Cucurbit[6]uril well defined complex of molecules held together by acts as a catalyst in cyclo-addition reactions. noncovalent bonds. Cucurbiturils are macrocyclic Detailed examination has been done on the molecules consisting of glycouril repeating units. construction of supramolecular organic, inorganic These supramolecular compounds are particularly compounds from macrocyclic cavitand interesting to chemists because they are molecular cucurbit[6]Uril and mono and polynuclear aqua containers that are capable of binding other complexes. Cucurbit[6]uril is used in the molecules within their cavities. -

Host-Guest Compounds Oligomeric Cucurbituril Complexes: from Peculiar Assemblies to Emerging Applications Xue Yang, Ruibing Wang, Anthony Kermagoret, David Bardelang
Host-Guest Compounds Oligomeric Cucurbituril Complexes: from Peculiar Assemblies to Emerging Applications Xue Yang, Ruibing Wang, Anthony Kermagoret, David Bardelang To cite this version: Xue Yang, Ruibing Wang, Anthony Kermagoret, David Bardelang. Host-Guest Compounds Oligomeric Cucurbituril Complexes: from Peculiar Assemblies to Emerging Applications. Ange- wandte Chemie International Edition, Wiley-VCH Verlag, 2020, pp.2-15. 10.1002/anie.202004622. hal-02972332 HAL Id: hal-02972332 https://hal-amu.archives-ouvertes.fr/hal-02972332 Submitted on 23 Oct 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Oligomeric Cucurbituril Complexes: from Peculiar Assemblies to Emerging Applications. Xue Yang,[a] Ruibing Wang,*[b] Anthony Kermagoret,*[a] and David Bardelang*[a] Abstract: Proteins are an endless source of inspiration. By carefully Xue Yang earned her Master’s degree from tuning the amino-acid sequence of proteins, nature made them evolve the University of Macau (China) in 2017. She from primary to quaternary structures, a property specific to protein is currently pursuing her PhD on the oligomers and often crucial to accomplish their function. On the other supramolecular chemistry of cucurbiturils at Aix- hand, the synthetic macrocycles cucurbiturils (CBs) have shown Marseille University. -

The X1 Method for Accurate and Efficient Prediction of Heats of Formation
THE JOURNAL OF CHEMICAL PHYSICS 127, 214105 ͑2007͒ The X1 method for accurate and efficient prediction of heats of formation ͒ Jianming Wu and Xin Xua State Key Laboratory of Physical Chemistry of Solid Surfaces and Center for Theoretical Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China ͑Received 31 July 2007; accepted 25 September 2007; published online 5 December 2007͒ We propose the X1 method which combines the density functional theory method with a neural network ͑NN͒ correction for an accurate yet efficient prediction of heats of formation. It calculates the final energy by using B3LYP/6-311+G͑3df,2p͒ at the B3LYP/6-311+G͑d,p͒ optimized geometry to obtain the B3LYP standard heats of formation at 298 K with the unscaled zero-point energy and thermal corrections at the latter basis set. The NN parameters cover 15 elements of H, Li, Be, B, C, N, O, F, Na, Mg, Al, Si, P, S, and Cl. The performance of X1 is close to the Gn theories, giving a mean absolute deviation of 1.43 kcal/mol for the G3/99 set of 223 molecules up to 10 nonhydrogen atoms and 1.48 kcal/mol for the X1/07 set of 393 molecules up to 32 nonhydrogen atoms. © 2007 American Institute of Physics. ͓DOI: 10.1063/1.2800018͔ I. INTRODUCTION the B3LYP method to calculate heats of formation of neutral organic molecules. Their results are promising. Calibrated ͑⌬ ͒ The accurate prediction of heats of formation Hf is with their own compilation of 180 organic molecules for the one of the central topics in computational chemistry. -
![Host-Guest Chemistry Between Cucurbit[7]Uril and Neutral and Cationic Guests](https://docslib.b-cdn.net/cover/2357/host-guest-chemistry-between-cucurbit-7-uril-and-neutral-and-cationic-guests-1532357.webp)
Host-Guest Chemistry Between Cucurbit[7]Uril and Neutral and Cationic Guests
HOST-GUEST CHEMISTRY BETWEEN CUCURBIT[7]URIL AND NEUTRAL AND CATIONIC GUESTS by Ian William Wyman A thesis submitted to the Department of Chemistry In conformity with the requirements for the degree of Doctor of Philosophy. Queen’s University Kingston, Ontario, Canada (January, 2010) Copyright © Ian William Wyman, 2010 Dedicated in memory of my Grandfather, Norman Brannen, 1917-2009. ii Abstract This thesis describes the host-guest chemistry between cucurbit[7]uril (CB[7]) and various series of guests, including neutral polar organic solvents, bis(pyridinium)alkane dications, local anaesthetics, acetylcholine analogues, as well as succinylcholine and decamethonium analogues, in aqueous solution. A focus of this thesis is the effects of varying the chemical structures within different series of guests upon the nature of the host-guest chemistry, such as the relative position and orientation of the guest relative to the CB[7] cavity, and the strengths of the binding affinities. The binding affinities of polar organic solvents with CB[7] depend upon the hydrophobic effect and dipole-quadrupole interactions. The polar guests align themselves so that their dipole moment is perpendicular to the quadrupole moment of CB[7]. The binding strengths of acetone and acetophenone to CB[7] decrease in the presence of alkali metals. Discrete 1:1 and 2:1 host-guest complexes are formed between CB[7] and a series of α,ω -bis(pyridinium)alkane guests. In most cases the CB[7] initially occupies the aliphatic linker when the 1:1 complex is formed and migrates to the terminal regions as the second CB[7] is added.