Chem 260 Handout 2013 Hydrocarbons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A-PDF Split DEMO : Purchase from to Remove the Watermark
A-PDF Split DEMO : Purchase from www.A-PDF.com to remove the watermark Figure 7.5 (a) Transition state for E2H-syn elimination. (b) Transition state for E2H-anti elimination. (c) Transition state for E,C elimination. tion state similar to that shown in Figure 7.5~is expected, since base attacks the molecule backside to the leaving group but frontside to the /3 hydrogen. Let us now turn to the experimental results to see if these predictions are borne out in fact. It has long been known that E2H reactions normally give preferentially anti elimination. For example, reaction of meso-stilbene dibromide with potassium ethoxide gives cis-bromostilbene (Reaction 7.39), whereas reaction of the D,L-dibromide gives the trans product (Reaction 7.40).lo2 A multitude of other examples exist-see, for example, note 64 (p. 355) and note 82 (p. 362). E2H reactions do, however, give syn elimination when: (1) an H-X di- hedral angle of 0" is achievable but one of 180" is not or, put another way, H and X can become syn-periplanar but not trans-periplanar ; (2) a syn hydrogen is much more reactive than the anti ones; (3) syn elimination is favored for steric reasons; and (4) an anionic base that remains coordinated with its cation, that, in turn, is coordinated with the leaving group, is used as catalyst. The very great importance of category 4 has only begun to be fully realized in the early 1970s. lo2 P. Pfeiffer, 2. Physik. Chem. Leibrig, 48, 40 (1904). 1,2-Elimination Reactions 371 An example of category 1 is found in the observation by Brown and Liu that eliminations from the rigid ring system 44, induced by the sodium salt of 2- cyclohexylcyclohexanol in triglyme, produces norborene (98 percent) but no 2-de~teronorbornene.~~~The dihedral angle between D and tosylate is O", but Crown ether present: No Yes that between H and tosylate is 120". -
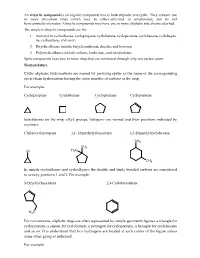
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

The Smallest Quantum Vortex
“The 2D Symmetry of Nature” T.M. Lach April 11, 2005 The structures of nature and the strong nuclear force The Proton the smallest Quantum Vortex Over the years many new discoveries have taught us that nature works its miracles with very simple structures. Going back 100 years we see this simplicity in structures like the Benzene molecule, Polycyclic Aromatic Hydrocarbons (PAH), the Hydrogen atom, DNA, snowflakes and crystal lattices. (1) In recent years many new discoveries, like the discovery of Quarks, have reinforced this belief. Ninety years ago, Niels Bohr’s model of the structure of the hydrogen atom (March 6 th 1913) revolutionized science since it brought together a few simple principles that allowed us to understand the nature of why atoms have quantized energy levels. Thus began the new era of Quantum Theory and Quantum Mechanics. Many before Bohr had assumed that the electrons were circling the nucleus. (2) Nicholson earlier had assumed that the angular momentum of the electron was quantized, for 30 years others had tried to explain the Balmer series of the hydrogen spectrum, but Bohr tied it all together into a coherent picture of the hydrogen atom. Bohr had to assume that the circling electrons did not lose energy to radiation and thereby spiral into the nucleus, a big assumption at the time. Ten years later in 1923 DeBroglie proposed the wave nature of particles, which explained why the circling electrons could maintain stable quantized orbits and so began the quantum nature of the universe, with a little help from Plank, Einstein, Shrodinger, Sommerfeld and many others. -

New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis
New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Author: Jason Joseph Marineau Persistent link: http://hdl.handle.net/2345/1741 This work is posted on eScholarship@BC, Boston College University Libraries. Boston College Electronic Thesis or Dissertation, 2010 Copyright is held by the author, with all rights reserved, unless otherwise noted. Boston College The Graduate School of Arts and Sciences Department of Chemistry NEW APPLICATIONS OF CYCLOBUTADIENE CYCLOADDITIONS: DIVERSITY AND TARGET ORIENTED SYNTHESIS a dissertation by JASON JOSEPH MARINEAU submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2010 © Copyright by JASON JOSEPH MARINEAU 2010 New Applications of Cyclobutadiene Cycloadditions: Diversity and Target Oriented Synthesis Jason J. Marineau Thesis Advisor: Professor Marc. L. Snapper Abstract Cyclobutadiene cycloadditions provide rapid access to rigid polycyclic systems with high strain energy and unusual molecular geometries. Further functionalization of these systems allows entry into unexplored chemical space. A tricarbonylcyclobutadiene iron complex on solid support enables exploration of these cycloadditions in a parallel format amenable to diversity oriented synthesis. Modeling of the cycloaddition transition states with density functional calculations provides a theoretical basis for analysis of the regioselectivity observed in generation of these substituted bicyclo[2.2.0]hexene derivatives. The high strain energy accessible in cyclobutadiene cycloadducts and their derivatives renders them useful synthons for access to medium-ring natural products through ring expansion. Torilin, a guaiane sesquiterpene isolated from extracts of the fruits of Torilis japonica, exhibits a range of biological activities including testosterone 5 -reductase inhibition, hKv1.5 channel blocking, hepatoprotective, anti-inflammatory and anti-cancer effects. -

1 Refinery and Petrochemical Processes
3 1 Refinery and Petrochemical Processes 1.1 Introduction The combination of high demand for electric cars and higher automobile engine effi- ciency in the future will mean less conversion of petroleum into fuels. However, the demand for petrochemicals is forecast to rise due to the increase in world popula- tion. With this, it is expected that modern and more innovative technologies will be developed to serve the growth of the petrochemical market. In a refinery process, petroleum is converted into petroleum intermediate prod- ucts, including gases, light/heavy naphtha, kerosene, diesel, light gas oil, heavy gas oil, and residue. From these intermediate refinery product streams, several fuels such as fuel gas, liquefied petroleum gas, gasoline, jet fuel, kerosene, auto diesel, and other heavy products such as lubricants, bunker oil, asphalt, and coke are obtained. In addition, these petroleum intermediates can be further processed and separated into products for petrochemical applications. In this chapter, petroleum will be introduced first. Petrochemicals will be intro- duced in the second part of the chapter. Petrochemicals – the main subject of this book – will address three major areas, (i) the production of the seven cornerstone petrochemicals: methane and synthesis gas, ethylene, propylene, butene, benzene, toluene, and xylenes; (ii) the uses of the seven cornerstone petrochemicals, and (iii) the technology to separate petrochemicals into individual components. 1.2 Petroleum Petroleum is derived from the Latin words “petra” and “oleum,” which means “rock” and “oil,” respectively. Petroleum also is known as crude oil or fossil fuel. It is a thick, flammable, yellow-to-black mixture of gaseous, liquid, and solid hydrocarbons formed from the remains of plants and animals. -

Dehydrogenation by Heterogeneous Catalysts
Dehydrogenation by Heterogeneous Catalysts Daniel E. Resasco School of Chemical Engineering and Materials Science University of Oklahoma Encyclopedia of Catalysis January, 2000 1. INTRODUCTION Catalytic dehydrogenation of alkanes is an endothermic reaction, which occurs with an increase in the number of moles and can be represented by the expression Alkane ! Olefin + Hydrogen This reaction cannot be carried out thermally because it is highly unfavorable compared to the cracking of the hydrocarbon, since the C-C bond strength (about 246 kJ/mol) is much lower than that of the C-H bond (about 363 kJ/mol). However, in the presence of a suitable catalyst, dehydrogenation can be carried out with minimal C-C bond rupture. The strong C-H bond is a closed-shell σ orbital that can be activated by oxide or metal catalysts. Oxides can activate the C-H bond via hydrogen abstraction because they can form O-H bonds, which can have strengths comparable to that of the C- H bond. By contrast, metals cannot accomplish the hydrogen abstraction because the M- H bonds are much weaker than the C-H bond. However, the sum of the M-H and M-C bond strengths can exceed the C-H bond strength, making the process thermodynamically possible. In this case, the reaction is thought to proceed via a three centered transition state, which can be described as a metal atom inserting into the C-H bond. The C-H bond bridges across the metal atom until it breaks, followed by the formation of the corresponding M-H and M-C bonds.1 Therefore, dehydrogenation of alkanes can be carried out on oxides as well as on metal catalysts. -

Here Science-Based Cost- Effective Pathways Forward?
Sponsors Institute of Atomic and Molecular Sciences, Academia Sinica, Taiwan PIRE-ECCI Program, UC Santa Barbara, USA Max-Planck-Gesellschaft, Germany National Science Council, Taiwan Organizing committee Dr. Kuei-Hsien Chen (IAMS, Academia Sinica, Taiwan) Dr. Susannah Scott (University of California - Santa Barbara, USA) Dr. Alec Wodtke (University of Göttingen & Max-Planck-Gesellschaft, Germany) Table of Contents General Information .............................................................................................. 1 Program for Sustainable Energy Workshop ....................................................... 3 I01 Dr. Alec M. Wodtke Beam-surface Scattering as a Probe of Chemical Reaction Dynamics at Interfaces ........................................................................................................... 7 I02 Dr. Kopin Liu Imaging the steric effects in polyatomic reactions ............................................. 9 I03 Dr. Chi-Kung Ni Energy Transfer of Highly Vibrationally Excited Molecules and Supercollisions ................................................................................................. 11 I04 Dr. Eckart Hasselbrink Energy Conversion from Catalytic Reactions to Hot Electrons in Thin Metal Heterostructures ............................................................................................... 13 I05 Dr. Jim Jr-Min Lin ClOOCl and Ozone Hole — A Catalytic Cycle that We Don’t Like ............... 15 I06 Dr. Trevor W. Hayton Nitric Oxide Reduction Mediated by a Nickel Complex -

Thermal Conductivity Correlations for Minor Constituent Fluids in Natural
Fluid Phase Equilibria 227 (2005) 47–55 Thermal conductivity correlations for minor constituent fluids in natural gas: n-octane, n-nonane and n-decaneଝ M.L. Huber∗, R.A. Perkins Physical and Chemical Properties Division, National Institute of Standards and Technology, Boulder, CO 80305, USA Received 21 July 2004; received in revised form 29 October 2004; accepted 29 October 2004 Abstract Natural gas, although predominantly comprised of methane, often contains small amounts of heavier hydrocarbons that contribute to its thermodynamic and transport properties. In this manuscript, we review the current literature and present new correlations for the thermal conductivity of the pure fluids n-octane, n-nonane, and n-decane that are valid over a wide range of fluid states, from the dilute-gas to the dense liquid, and include an enhancement in the critical region. The new correlations represent the thermal conductivity to within the uncertainty of the best experimental data and will be useful for researchers working on thermal conductivity models for natural gas and other hydrocarbon mixtures. © 2004 Elsevier B.V. All rights reserved. Keywords: Alkanes; Decane; Natural gas constituents; Nonane; Octane; Thermal conductivity 1. Introduction 2. Thermal conductivity correlation Natural gas is a mixture of many components. Wide- We represent the thermal conductivity λ of a pure fluid as ranging correlations for the thermal conductivity of the lower a sum of three contributions: alkanes, such as methane, ethane, propane, butane and isobu- λ ρ, T = λ T + λ ρ, T + λ ρ, T tane, have already been developed and are available in the lit- ( ) 0( ) r( ) c( ) (1) erature [1–6]. -

Measurements of Higher Alkanes Using NO Chemical Ionization in PTR-Tof-MS
Atmos. Chem. Phys., 20, 14123–14138, 2020 https://doi.org/10.5194/acp-20-14123-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Measurements of higher alkanes using NOC chemical ionization in PTR-ToF-MS: important contributions of higher alkanes to secondary organic aerosols in China Chaomin Wang1,2, Bin Yuan1,2, Caihong Wu1,2, Sihang Wang1,2, Jipeng Qi1,2, Baolin Wang3, Zelong Wang1,2, Weiwei Hu4, Wei Chen4, Chenshuo Ye5, Wenjie Wang5, Yele Sun6, Chen Wang3, Shan Huang1,2, Wei Song4, Xinming Wang4, Suxia Yang1,2, Shenyang Zhang1,2, Wanyun Xu7, Nan Ma1,2, Zhanyi Zhang1,2, Bin Jiang1,2, Hang Su8, Yafang Cheng8, Xuemei Wang1,2, and Min Shao1,2 1Institute for Environmental and Climate Research, Jinan University, 511443 Guangzhou, China 2Guangdong-Hongkong-Macau Joint Laboratory of Collaborative Innovation for Environmental Quality, 511443 Guangzhou, China 3School of Environmental Science and Engineering, Qilu University of Technology (Shandong Academy of Sciences), 250353 Jinan, China 4State Key Laboratory of Organic Geochemistry and Guangdong Key Laboratory of Environmental Protection and Resources Utilization, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, 510640 Guangzhou, China 5State Joint Key Laboratory of Environmental Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, 100871 Beijing, China 6State Key Laboratory of Atmospheric Boundary Physics and Atmospheric Chemistry, Institute of Atmospheric Physics, Chinese -

Development of Health-Based Alternative to the Total Petroleum Hydrocarbon (Tph) Parameter
INTERIM FINAL PETROLEUM REPORT: DEVELOPMENT OF HEALTH-BASED ALTERNATIVE TO THE TOTAL PETROLEUM HYDROCARBON (TPH) PARAMETER Prepared for: Bureau of Waste Site Cleanup Massachusetts Department of Environmental Protection Boston, MA Prepared by: Office of Research and Standards Massachusetts Department of Environmental Protection Boston, MA and ABB Environmental Services, Inc. Wakefield, MA AUGUST 1994 INTERIM FINAL PETROLEUM REPORT: DEVELOPMENT OF HEALTH-BASED ALTERNATIVE TO THE TOTAL PETROLEUM HYDROCARBON (TPH) PARAMETER TABLE OF CONTENTS Section Title Page No. EXECUTIVE SUMMARY .................................................................................................. v AUTHORS AND REVIEWERS .......................................................................................... ix 1.0 INTRODUCTION.......................................................................................................1-1 1.1 Background ...............................................................................................1-1 1.2 Purpose......................................................................................................1-2 1.3 Approach ...................................................................................................1-6 2.0 HAZARD IDENTIFICATION FOR PETROLEUM HYDROCARBONS.....................2-1 2.1 Composition of Petroleum Products...........................................................2-1 2.2 Toxic Effects of Whole Products................................................................2-5 2.2.1 Gasoline.........................................................................................2-5 -

2. Alkanes and Cycloalkanes
BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H. Hart, L. E. Craine, D. J. Hart, C. M. Hadad, 4) Organic Chemistry: A Brief Course, R. C. Atkins, F.A. Carey Hazırlayanlar : Prof. Dr. Pervin Ünal Civcir ve Doç. Dr. Melike Kalkan 2. ALKANES AND CYCLOALKANES 2.1 Nomenclature of alkanes 2.2 Physical Properties of Alkanes 2.3 Preparation of Alkanes 2.3.1 Hydrogenation of Alkenes and Alkynes 2.3.2 Alkanes From Alkyl Halides 2.3.2.1 Reduction 2.3.2.2 Wurtz Reaction 2.3.2.3 Hydrolysis of Grignard Reagent 2.3.3 Reduction of Carbonyl Compounds 2.3.3.1 Clemmensen Reduction 2.3.3.2 Wolf- Kıschner Reduction 2.3.3.3 Alkanes from Carboxylic Acids 2.4 Reactions of Alkanes 2.4.1 Radicalic substitution reactions 2.4.2 Combustion Reactions 2.4.3 Nitration 2.4.4 Cracking 2.5 Cycloalkanes 2.5.1 Nomenclature of Cycloalkanes 2.5.2 Conformations of Cycloalkanes 2.5.3 Substituted Cycloalkanes 2.5.3.1 Monosubstitued Cycloalkanes 2.5.3.2 Disubstitued Cycloalkanes Hazırlayanlar : Prof. Dr. Pervin Ünal Civcir ve Doç. Dr. Melike Kalkan 2.1 Nomenclature of Alkanes Alkanes with increasing numbers of carbon atoms have names are based on the Latin word for the number of carbon atoms in the chain of each molecule. Nomenclature of Straight Chain Alkanes: n CnH2n+2 n-Alkane n CnH2n+2 n-Alkane n CnH2n+2 n-Alkane 1 CH4 methane 6 C6H14 hexane 11 C11H24 undecane 2 C2H6 ethane 7 C7H16 heptane 12 C12H26 dodecane 3 C3H8 propane 8 C8H18 octane 13 C13H28 tridecane 4 C4H10 butane 9 C9H20 nonane 14 C14H30 tetradecane 5 C5H12 pentane 10 C10H22 decane 15 C15H32 pentadecane 2.2 Physical Properties of Alkanes (i) The first four alkanes are gases at room temperature. -

(C2–C8) Sources and Sinks Around the Arabian Peninsula
Atmos. Chem. Phys., 19, 7209–7232, 2019 https://doi.org/10.5194/acp-19-7209-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Non-methane hydrocarbon (C2–C8) sources and sinks around the Arabian Peninsula Efstratios Bourtsoukidis1, Lisa Ernle1, John N. Crowley1, Jos Lelieveld1, Jean-Daniel Paris2, Andrea Pozzer1, David Walter3, and Jonathan Williams1 1Department of Atmospheric Chemistry, Max Planck Institute for Chemistry, Mainz 55128, Germany 2Laboratoire des Sciences du Climat et de l’Environnement, CEA-CNRS-UVSQ, UMR8212, IPSL, Gif-sur-Yvette, France 3Department of Multiphase Chemistry, Max Planck Institute for Chemistry, Mainz 55128, Germany Correspondence: Efstratios Bourtsoukidis ([email protected]) Received: 29 January 2019 – Discussion started: 14 February 2019 Revised: 25 April 2019 – Accepted: 29 April 2019 – Published: 29 May 2019 Abstract. Atmospheric non-methane hydrocarbons source-dominated areas of the Arabian Gulf (bAG D 0:16) (NMHCs) have been extensively studied around the and along the northern part of the Red Sea (bRSN D 0:22), but globe due to their importance to atmospheric chemistry stronger dependencies are found in unpolluted regions such and their utility in emission source and chemical sink as the Gulf of Aden (bGA D 0:58) and the Mediterranean Sea identification. This study reports on shipborne NMHC (bMS D 0:48). NMHC oxidative pair analysis indicated that measurements made around the Arabian Peninsula during OH chemistry dominates the oxidation of hydrocarbons in the AQABA (Air Quality and climate change in the Arabian the region, but along the Red Sea and the Arabian Gulf the BAsin) ship campaign.