Cyclophane. Do MM Calculations of Each of These
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2004 DOE/BES Analysis Program Contractors' Meeting
2004 DOE/BES Analysis Program Contractors’ Meeting Annapolis, Maryland February 12 – 14, 2004 Sponsored by The U.S. Department of Energy Office of Basic Energy Sciences Workshop Chair: John Miller 2004 DOE/BES Analysis Program Contractors’ Meeting Program and Abstracts Department of Energy Office of Science Office of Basic Energy Sciences Chemical Sciences, Geosciences and Biosciences Division FOREWORD This abstract booklet provides a record of the 2004 U.S. Department of Energy, Office of Basic Energy Sciences, Analysis Program Contractors’ Meeting. This group of scientists last met as part of the larger Separations and Analysis Program Contractors’ Meeting held in San Diego April 5-7, 2001. The agenda and abstracts of that meeting may be found on the web at http://www.sc.doe.gov/bes/chm/Publications/publications.html. There is wide agreement that a gathering of researchers with common interests and sponsorship provides a fruitful environment for exchange of research results, research techniques, and research opportunities. The primary means of communicating research achievements and perspectives at this meeting is oral presentations, formal discussion periods and informal breaks and meals. The agenda has been organized so that papers in related disciplines – such as mass spectrometry or optical spectroscopy – are loosely clustered together. I am pleased to have the privilege of organizing this meeting and of serving as the program manager of this world-class research program. In carrying out these tasks, I learn from the achievements, and share the excitement, of the research of the many sponsored scientists and students whose names appear on the papers in the following pages. -
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
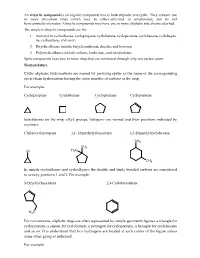
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

The Smallest Quantum Vortex
“The 2D Symmetry of Nature” T.M. Lach April 11, 2005 The structures of nature and the strong nuclear force The Proton the smallest Quantum Vortex Over the years many new discoveries have taught us that nature works its miracles with very simple structures. Going back 100 years we see this simplicity in structures like the Benzene molecule, Polycyclic Aromatic Hydrocarbons (PAH), the Hydrogen atom, DNA, snowflakes and crystal lattices. (1) In recent years many new discoveries, like the discovery of Quarks, have reinforced this belief. Ninety years ago, Niels Bohr’s model of the structure of the hydrogen atom (March 6 th 1913) revolutionized science since it brought together a few simple principles that allowed us to understand the nature of why atoms have quantized energy levels. Thus began the new era of Quantum Theory and Quantum Mechanics. Many before Bohr had assumed that the electrons were circling the nucleus. (2) Nicholson earlier had assumed that the angular momentum of the electron was quantized, for 30 years others had tried to explain the Balmer series of the hydrogen spectrum, but Bohr tied it all together into a coherent picture of the hydrogen atom. Bohr had to assume that the circling electrons did not lose energy to radiation and thereby spiral into the nucleus, a big assumption at the time. Ten years later in 1923 DeBroglie proposed the wave nature of particles, which explained why the circling electrons could maintain stable quantized orbits and so began the quantum nature of the universe, with a little help from Plank, Einstein, Shrodinger, Sommerfeld and many others. -

Synthetic Applications of Dienes and Polyenes, Excluding Cycloadditions
The Chemistry of Dienes and Polyenes. Volume 2 Edited by Zvi Rappoport Copyright 2000 John Wiley & Sons, Ltd. ISBN: 0-471-72054-2 CHAPTER 9 Synthetic applications of dienes and polyenes, excluding cycloadditions NANETTE WACHTER-JURCSAK Department of Chemistry, Biochemistry and Natural Science, Hofstra University, Hempstead, New York 11549-1090, USA Fax: (516) 463-6394; e-mail: [email protected] and KIMBERLY A. CONLON Department of Pharmacological Sciences, School of Medicine, State University of New York at Stony Brook, Stony Brook, New York 11794-8651, USA Fax: (516) 444-3218; e-mail: [email protected] I. INTRODUCTION ..................................... 693 II. ADDITION REACTIONS ............................... 694 III. OXIDATION REACTIONS ............................... 700 IV. COUPLING REACTIONS ............................... 710 A. Wittig Reactions of Dienes and Polyenes .................... 711 B. Coupling Promoted by Organometallic Reagents ............... 712 V. DIMERIZATION REACTIONS ............................ 718 VI. PREPARATION OF METAL–POLYENE COMPLEXES ........... 720 VII. REARRANGEMENTS .................................. 722 A. Cope Rearrangement ................................. 722 B. Claisen Rearrangement ............................... 728 VIII. REFERENCES ....................................... 736 I. INTRODUCTION The reactivity of polyenes is influenced by their substituents, and whether or not the multiple double bonds of the unsaturated hydrocarbon are conjugated or isolated from 693 694 Nanette Wachter-Jurcsak and Kimberly A. Conlon one another. The -system of a polyene may be fully conjugated, or there may be one or more pairs of conjugated double bonds isolated from the other -bonds in the molecule, or, alternatively, each of the carbon–carbon double bonds in the polyene may be isolated from one another. Conjugated -systems react differently with electrophiles than isolated double bonds. Addition of hydrogen to isolated double bonds has been previously discussed in this series and will not be addressed here1. -

Curriculum Vitae
1 CURRICULUM VITAE Robert Bau Born: February 10, 1944, Shanghai, China (naturalized U.S. citizen, 1974) Education: B.Sc., University of Hong Kong, June, 1964 Ph.D., University of California at Los Angeles, March, 1968 ` Postdoctoral Research Fellow, Harvard University, 1968-69 Positions Held Since Ph.D. Degree: 1977-present Professor of Chemistry, University of Southern California 1974-77 Associate Professor of Chemistry, University of Southern California 1969-74 Assistant Professor of Chemistry, University of Southern California Awards and Honors: Fellow of the Alfred P. Sloan Foundation, 1974-76 NIH Research Career Development Awardee, 1975-80 Recipient of USC Associates Award for Excellence in Teaching, 1974 Recipient of USC Associates Award for Excellence in Research, 1979 Fellow of the American Association for the Advancement of Science, 1982 Recipient of the Alexander van Humboldt Foundation, U.S. Senior Scientist Award, 1985 Visiting Professor of Chemistry, University of Grenoble, France, March-June, 1989 President, American Crystallographic Association, 2006 Research Interests: X-ray and Neutron Diffraction Studies of Covalent Metal Hydride Compounds Neutron Diffraction Studies on Molecules Having Chiral Methylene Groups (Molecules of the type CHDRR') Neutron Diffraction Studies of Small Proteins 2 PUBLICATION LIST Professor Robert Bau Department of Chemistry University of Southern California Los Angeles, California 90089-0744 1. "The Crystal Structure of HRe2Mn(CO)14. A Neutral, 'Electron Deficient', Polynuclear Carbonyl Hydride", H.D. Kaesz, R. Bau, and M.R. Churchill, J. Am. Chem. Soc., 89, 2775 (1967). 2. "Spectroscopic Studies of Isotopically Substituted Metal Carbonyls. I. Vibrational Analysis of Metal Pentacarbonyl Halides", H.D. Kaesz, R. Bau, D. Hendrickson and J.M. -

Synthetic Advances in the C‐H Activation of Rigid Scaffold Molecules
Synthesis Review Synthetic Advances in the C‐H Activation of Rigid Scaffold Molecules Nitika Grovera Mathias O. Senge*a a School of Chemistry, Trinity College Dublin, The University of Dublin, Trinity Biomedical Sciences Institute, 152–160 Pearse Street, Dublin 2, Ireland [email protected] Dedicated to Prof. Dr. Henning Hopf Received: only recall the epic endeavors involved in Eaton’s cubane Accepted: Published online: synthesis,3 Parquette’s preparation of dodecahedrane, DOI: Prinzbach’s pagodane route thereto, or Maier’s synthesis of Abstract The remarkable structural and electronic properties of rigid non‐ tetra-tert-butyltetrahedrane.4 conjugated hydrocarbons afford attractive opportunities to design molecular building blocks for both medicinal and material applications. The bridgehead positions provide the possibility to append diverse functional groups at specific angles and in specific orientations. The current review summarizes the synthetic development in CH functionalization of the three rigid scaffolds namely: (a) cubane, (b) bicyclo[1.1.1]pentane (BCP), (c) adamantane. 1 Introduction 2 Cubane 2.1 Cubane Synthesis 2.2 Cubane Functionalization 3 BCP 3.1 BCP Synthesis 3.2 BCP Functionalization 4 Adamantane 4.1 Adamantane Synthesis 4.2 Adamantane Functionalization 5 Conclusion and Outlook Key words Cubane, bicyclo[1.1.1]pentane, adamantane, rigid scaffolds, CH‐ functionalization. Figure 1 The structures of cubane, BCP and adamantane and the five platonic hydrocarbon systems. The fascinating architecture of these systems significantly 1 Introduction differs from scaffolds realized in natural compounds. Hence, they attracted considerable attention from synthetic and The practice of synthetic organic chemistry of non-natural physical organic chemists to investigate their properties and to compounds with rigid organic skeletons provides a deep establish possible uses.3,5 Significantly, over the past twenty understanding of bonding and reactivity. -

Nomenclature – Naming of Organic Compounds
12/05/2020 Nomenclature –Naming of Organic Compounds 1 ‘Trivial’ Names… Aspirin Cubane Housane Basketane Olympicene 2 1 12/05/2020 IUPAC International Union of Pure and Applied Chemistry Pentacyclo[4.2.0.02,5.03,8.04,7]octane 3 Naming Organic Compounds Chains meth 1 C eth 2 C C prop 3 C C C but 4 CCCC pent 5 C C C C C hex 6 C C C C C C hept 7 C C C C C C C oct 8 C C C C C C C C non 9 C C C C C C C C C 4 2 12/05/2020 Chains – Numbering Positions of Substituents Cl C C C C C C C 2‐chloro… 1234567 Cl C C C C C C C 3‐chloro… 7 6 5 4 3 2 1 5 Single and Multiple Carbon‐Carbon Bonds in Chains C C C C C C Single carbon‐ Contains a Contains a an carbon bonds en carbon‐carbon yn carbon‐carbon only double bond triple bond C C C C C C C …‐1‐ene 1234567 C C C C C C C …‐2‐ene 1 234567 C C C C C C C …‐3‐ene 7 6 5 4 3 2 1 6 3 12/05/2020 Branches to Carbon Chains H H C CH3 methyl H Common H H branches H C C CH CH ethyl on chains: 3 2 H H H H H H C C C CH3 CH2CH2 propyl H H H 7 Position (3‐) Branch (methyl) Longest chain HHCH HHHH Length of longest chain (7 = hept) 3 Only single C‐C bonds (ane) H C C C C C C C H 1234567 3‐methylheptane HHHHHHH HHHHH H C C C C C H 3 4 5 6 HHHH 3‐methylhexane H C 2 H Longest chain H C1 H H 2‐methylhex‐2‐ene 8 4 12/05/2020 For each of the alkanes below, identify and highlight the longest unbroken chain. -

Cyclopentane Synthesis
Cyclopentane Synthesis Dan O’Malley Baran Group Meeting Cyclopentane Synthesis Group Meeting O'Malley 2/9/2005 This presentation is broken down into the following catagories. Some reactions either fit more than one Students of organic chemistry are taught a number of reactions for the synthesis of category or do not fit easily into any of them. Efforts have been made to place all such reactions in the cyclohexanes at a very early stage of their careers. Techniques for the creation of cyclopentanes, most appropriate category. however, are generally taught at a much later stage and are rarely given the same detailed treatment. This may be the result of the fact that there are no equivalents of reactions such as the Diels-Alder and I. General Information Robinson Annulation in terms of generality, extent of use, and historical importance. This may, in turn, II. Ionic Reactions be caused by the fact that the cyclopentane is an inherintly "umpoled" functionality, as illustrated below. III. Metal Mediated Reactions IV. Radical Reactions FG V. Pericyclic and Pseudo-pericyclic Reactions VI. Ring Expansion and Contraction Reactions I. General Information This situation is further exacerbated by the general lack of cheaply available cyclopentane compounds Baldwin's rules in the chiral pool; wheras a number of cyclohexane terpenes are readily available for elaboration, there Baldwin has divided ring closure reactions into those that are "favored" and those that are "disfavored". are no analogous cylcopentane natural products. Cyclopentanes are however, present in many Those that are disfavored are not always impossible, but are frequently much more difficult to effect. -

Chem 51A Chapter 4 2014
Lecture Notes Chem 51A S. King Chapter 4 Alkanes I. Alkanes – An Introduction Alkanes are aliphatic hydrocarbons having only C−H and C−C σ-bonds. They can be cyclic or acyclic. • Acyclic alkanes have the molecular formula CnH2n+2 (where n = an integer.) They are also called saturated hydrocarbons because they have the maximum number of hydrogen atoms per carbon. • Cyclic alkanes contain carbon atoms joined into a ring. They have molecular formula CnH2n. (Notice: 2 less hydrogens than saturated alkanes – Why? A. Unbranched Alkanes Alkanes with unbranched carbon chains are also known as normal alkanes or n- alkanes. The first four n-alkanes: H H H H H H H H H H H C H H C C H H C C C H H C C C C H H H H H H H H H H H • This is an example of a family of compounds known as homologous series (each member differs from the next by the addition of one methylene group (CH2). The first 12 n-alkanes are listed in the handout Essential Nomenclature. 71 Look at the various representations of propane: H H H H H H H C3H8 CH3CH2CH3 H C C C H C C H C H H H H H H H CH 3 H CH3 C H H H CH3 H H B. Branched Alkanes As the number of carbons of an alkane increase beyond three, the number of possible structures increases. An alkane with molecular formula C4H10, for example has two different ways to connect atoms together: CH3 CH2 CH2 CH3 CH3 CH CH3 CH3 These are examples of constitutional isomers: Compounds that have the same molecular formula but different connectivity. -

Chem 260 Handout 2013 Hydrocarbons
Hydrocarbons — Compounds that contain only Carbon and Hydrogen Types of hydrocarbons: Saturated: Alkanes only single, covalent C-C and C-H bonds, no rings Cycloalkanes same, but contain rings Unsaturated: Alkenes contain > 1 C=C double bond Alkynes contain > 1 C≡C triple bond Aromatic contain > 1 benzene ring General formula for alkanes is: CnH2n+2, n = 1, 2, 3 ... All carbons in alkanes are sp3 hybridized and tetrahedral (bond angles are 109.5°). The suffix "-ane" denotes an alkane. Be familiar with the variety of types of drawings of organic molecules. Know what atom is bonded to what atom. Dashes/wedges: recall that dashed bonds are going into the page, wedged bonds are coming out of the page, and lines are in the plane of the page. Line drawings: Each intersection or end of a line represents a carbon with the correct number of hydrogen atoms. Carbon always has four bonds in stable species. Hydrogens are often not drawn: be sure you know how many hydrogens are on each carbon: draw them in each time if it helps you. Be familiar with the following nomenclature: n = 1 methane n = 5 pentane n = 8 octane n = 2 ethane n = 6 hexane n = 9 nonane n = 3 propane n = 7 heptane n = 10 decane n = 4 butane Interlude: What do we have to know? Will this be on the exam? Anything could appear on an exam. But you should focus on what you really need to know. Know the drawings and nomenclature (don’t bother with index cards, most nomenclature comes just be doing homework). -

NBO Applications, 2005
NBO 2005 – 686 references Abbati, G. L.; Aragoni, M. C.; Arca, M.; Carrea, M. B.; Devillanova, F. A.; Garau, A.; Isaia, F.; Lippolis, V.; Marcelli, M.; Silvestru, C.; Verani, G. Gold(0) and gold(III) reactivity towards the tetraphenyldithioimidodiphosphinic acid, [Ph2P(S)NHP(S)Ph-2] European Journal of Inorganic Chemistry: 589-596 2005. Abirami, S.; Xing, Y. M.; Tsang, C. W.; Ma, N. L. Theoretical study of alpha/beta-alanine and their protonated/alkali metal cationized complexes Journal of Physical Chemistry A, (109): 500-506 2005. Adcock, W.; Graton, J.; Laurence, C.; Lucon, M.; Trout, N. Three-centre hydrogen bonding in the complexes of syn-2,4-difluoroadamantane with 4- fluorophenol and hydrogen fluoride Journal of Physical Organic Chemistry, (18): 227-234 2005. Afanas'yev, D. Y.; Prosyanik, A. V. Why N-methyleneformamide, CH2=N-CHO, prefers gauche-conformation? A DFT/NBO study Journal of Molecular Structure-Theochem, (130): 45-49 2005. Agou, T.; Kobayashi, J.; Kawashima, T. Dibenzophosphaborin: A hetero-pi-conjugated molecule with fluorescent properties based on intramolecular charge transfer between phosphorus and boron atoms Organic Letters, (7): 4373-4376 2005. Ahlquist, M.; Fabrizi, G.; Cacchi, S.; Norrby, P. O. Palladium(0) alkyne complexes as active species: a DFT investigation Chemical Communications: 4196-4198 2005. Alajarin, M.; Ortin, M. M.; Sanchez-Andrada, P.; Vidal, A.; Bautista, D. From ketenimines to ketenes to quinolones: Two consecutive pseudopericyclic events Organic Letters, (7): 5281-5284 2005. Alajarin, M.; Sanchez-Andrada, P.; Lopez-Leonardo, C.; Alvarez, A. On the mechanism of phthalimidine formation via o-phthalaldehyde monoimines. New [1,5]-H sigmatropic rearrangements in molecules with the 5-aza-2,4-pentadienal Journal of Organic Chemistry, (70): 7617-7623 2005. -

4.3.6 Strain Energies and Heats of Formation
Results and Discussion • 183 ∆ –1 dramatically reduces the barrier to inversion ( Eplan drops from 58.6 to 7.4 kJ mol ). This might partly reflect the dramatic reduction in the energy difference between ‘pla- ∆ nar’ and tetrahedral-like structures for neopentane and spiropentane ( EPT = 880 and 440 kJ mol–1, respectively) (see Section 4.3.2). The introduction of a pair of methylene bridges between the caps then reduces this barrier to zero, giving a broadened potential energy well with an equilibrium structure with D2h symmetry. 4.3.6 Strain Energies and Heats of Formation Determining the total strain energies (SEs) of our novel hydrocarbons allows for a comparison with other strained hydrocarbons.43 We have chosen to use a method of cal- culating strain energies which has been used to great effect by Schulman and Disch.32 This method determines the strain energy as the negative of the calculated enthalpy change of a homodesmic reaction in which the number of quaternary (C), tertiary (CH) and secondary (CH2) carbons present in the target hydrocarbon are balanced with prod- uct neopentane, isobutane and propane molecules. The number of primary (CH3) car- bons on each side of the reaction is then balanced using ethane. This preserves the number and type of C–C bonds on each side of the reaction and is found to give good cancellation of errors when the MP2 method is used to calculate energies (see also Section 3.2 on page 97). If these product molecules are defined as being strain free (which is usual), the enthalpy change of this homodesmic reaction gives the total strain of the target hydrocarbon.