Cyclopentane Synthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2004 DOE/BES Analysis Program Contractors' Meeting
2004 DOE/BES Analysis Program Contractors’ Meeting Annapolis, Maryland February 12 – 14, 2004 Sponsored by The U.S. Department of Energy Office of Basic Energy Sciences Workshop Chair: John Miller 2004 DOE/BES Analysis Program Contractors’ Meeting Program and Abstracts Department of Energy Office of Science Office of Basic Energy Sciences Chemical Sciences, Geosciences and Biosciences Division FOREWORD This abstract booklet provides a record of the 2004 U.S. Department of Energy, Office of Basic Energy Sciences, Analysis Program Contractors’ Meeting. This group of scientists last met as part of the larger Separations and Analysis Program Contractors’ Meeting held in San Diego April 5-7, 2001. The agenda and abstracts of that meeting may be found on the web at http://www.sc.doe.gov/bes/chm/Publications/publications.html. There is wide agreement that a gathering of researchers with common interests and sponsorship provides a fruitful environment for exchange of research results, research techniques, and research opportunities. The primary means of communicating research achievements and perspectives at this meeting is oral presentations, formal discussion periods and informal breaks and meals. The agenda has been organized so that papers in related disciplines – such as mass spectrometry or optical spectroscopy – are loosely clustered together. I am pleased to have the privilege of organizing this meeting and of serving as the program manager of this world-class research program. In carrying out these tasks, I learn from the achievements, and share the excitement, of the research of the many sponsored scientists and students whose names appear on the papers in the following pages. -

Lewis Acid Mediated Reactions of Olefins with Carbonyls
Lewis Acid Mediated Reactions of Olefins with Carbonyls Submitted by Stephen Flower For the Degree of PhD Of the University of Bath 2002 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be published without the prior written consent of the author. This thesis may be made available for consultation within the University Library and may be photocopied or lent to other libraries for the purposes of consultation. …………………………..(signed) …………………………..(date) Abstract This thesis is divided into 5 chapters. The first chapter reviews the literature of Carbonyl-ene and Prins reactions. Recent advances in these areas and their application to natural product synthesis and other targeted syntheses are discussed. The second chapter discusses the concept of desymmetrisation using selected examples, including desymmetrising Carbonyl-ene reactions. Chapter Three introduces and discusses the work undertaken in studying the desymmetrising intramolecular carbonyl-ene of a meso-dialdehyde. Chapter Four details the concept of pyruvate-Prins cyclisation giving examples of its potential use. The chapter also gives examples of the use of rigid stereodefined scaffolds and their application. The chapter describes the reactivity of substituted pyruvate esters with cyclic enol ethers, and the elaboration of the cyclised products. The fifth chapter provides experimental details of the procedures reported. i Acknowledgements Many thanks to Dr Mike Willis for all his help and guidance and boundless enthusiasm over the years; and for a detailed investigation of the local pubs. -

BI(III) Initiated Cyclization Reactions and Iodonium Fragmentation Kinetics
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2005 BI(III) Initiated Cyclization Reactions and Iodonium Fragmentation Kinetics Yajing Lian College of William & Mary - Arts & Sciences Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Inorganic Chemistry Commons Recommended Citation Lian, Yajing, "BI(III) Initiated Cyclization Reactions and Iodonium Fragmentation Kinetics" (2005). Dissertations, Theses, and Masters Projects. Paper 1539626842. https://dx.doi.org/doi:10.21220/s2-zj7g-fp23 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. BI(III) INITIATED CYCLIZATION REACTIONS AND IODONIUM FRAGMENTATION KINETICS A Thesis Presented to The Faculty of the Department of Chemistry The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Science by Yajing Lian 2005 APPROVAL SHEET This thesis is submitted in partial fulfillment of The requirements for the degree of Master of Science A Yajing Lian Approved by the Committee, December 20, 2005 Robert J. Hinkle, Chair Christopher Jl A] Robert D. Pike TABLE OF CONTENTS Page Acknowledgements VI List of Tables Vll List of Schemes V lll List of Figures IX Abstract XI Introduction Chapter I Secondary -

Deuterium Exchange Studies of Some Cyclopentenone Derivatives Robert Logan Myers Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1963 Deuterium exchange studies of some cyclopentenone derivatives Robert Logan Myers Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Myers, Robert Logan, "Deuterium exchange studies of some cyclopentenone derivatives " (1963). Retrospective Theses and Dissertations. 2549. https://lib.dr.iastate.edu/rtd/2549 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 64—3885 microfilmed exactly as received MYERS, Robert Logan, 1937- DEUTERIUM EXCHANGE STUDIES OF SOME CYCLOPENTENONE DERIVATIVES. Iowa State University of Science and Technology Ph.D., 1963 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan DEUTERIUM EXCHANGE STUDIES OF SOME CYCIOPEHTENONE DERIVATIVES Robert Logan Myers A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Organic Chemistry Approved : Signature was redacted for privacy. Work Signature was redacted for privacy. Head of Major Department Signature was redacted for privacy. Dean Iowa State University Of Science and Technology Ames, Iowa 1963 11 TABLE OF CONTENTS Page INTRODUCTION 1 HISTORICAL 3 DISCUSSION 1Ç EXPERIMENTAL 43 SUMMARY 82 ACKNOWLEDGEMENTS 83 APPENDIX 84 1 INTRODUCTION Synthetic methods for the preparation of highly substi tuted 5-benzylidenecyclopentenones have long been known. -

Synthetic Applications of Dienes and Polyenes, Excluding Cycloadditions
The Chemistry of Dienes and Polyenes. Volume 2 Edited by Zvi Rappoport Copyright 2000 John Wiley & Sons, Ltd. ISBN: 0-471-72054-2 CHAPTER 9 Synthetic applications of dienes and polyenes, excluding cycloadditions NANETTE WACHTER-JURCSAK Department of Chemistry, Biochemistry and Natural Science, Hofstra University, Hempstead, New York 11549-1090, USA Fax: (516) 463-6394; e-mail: [email protected] and KIMBERLY A. CONLON Department of Pharmacological Sciences, School of Medicine, State University of New York at Stony Brook, Stony Brook, New York 11794-8651, USA Fax: (516) 444-3218; e-mail: [email protected] I. INTRODUCTION ..................................... 693 II. ADDITION REACTIONS ............................... 694 III. OXIDATION REACTIONS ............................... 700 IV. COUPLING REACTIONS ............................... 710 A. Wittig Reactions of Dienes and Polyenes .................... 711 B. Coupling Promoted by Organometallic Reagents ............... 712 V. DIMERIZATION REACTIONS ............................ 718 VI. PREPARATION OF METAL–POLYENE COMPLEXES ........... 720 VII. REARRANGEMENTS .................................. 722 A. Cope Rearrangement ................................. 722 B. Claisen Rearrangement ............................... 728 VIII. REFERENCES ....................................... 736 I. INTRODUCTION The reactivity of polyenes is influenced by their substituents, and whether or not the multiple double bonds of the unsaturated hydrocarbon are conjugated or isolated from 693 694 Nanette Wachter-Jurcsak and Kimberly A. Conlon one another. The -system of a polyene may be fully conjugated, or there may be one or more pairs of conjugated double bonds isolated from the other -bonds in the molecule, or, alternatively, each of the carbon–carbon double bonds in the polyene may be isolated from one another. Conjugated -systems react differently with electrophiles than isolated double bonds. Addition of hydrogen to isolated double bonds has been previously discussed in this series and will not be addressed here1. -

Mass Spectrometric Research of Hydrogenated Molecules of Carbon As Products of Pyrolysis of Benzene and Pyridine Vapours
Chemical and Materials Engineering 1(4): 122-131, 2013 http://www.hrpub.org DOI: 10.13189/cme.2013.010404 Mass Spectrometric Research of Hydrogenated Molecules of Carbon as Products of Pyrolysis of Benzene and Pyridine Vapours Alexey Kharlamov1, Marina Bondarenko1,*, Ganna Kharlamova2 1Frantsevich Institute for Problems of Materials Science of NASU, Krzhyzhanovsky St. 3, 03680 Kiev, Ukraine 2Taras Shevchenko National University of Kiev, Volodymyrs'ka St. 64, 01601 Kiev, Ukraine *Corresponding Author: [email protected] Copyright © 2013 Horizon Research Publishing All rights reserved. Abstract Hydrogenated carbon molecules are to speak about creation on the basis of system of convertible synthesized by a method which essentially is distinct from reactions already known methods of preparation of fulleranes as this C60+30H2 C60H60 (1) method a preliminary stage of synthesis of carbon molecules accumulator of hydrogen with so huge (7.7mass. %) contents is excluded completely. Fulleranes and quasi-fulleranes as ⇌ of hydrogen. Though here it is necessary to note, that in nanodimentional particles were in common deposited by dodecahedrane С Н , which was synthesized by Paquette ethanol from benzene-xylene extracts from products of 20 20 [4] three years prior to opening of fullerene С (and 8 years pyrolysis of vapours of benzene and pyridine. The 60 prior to obtaining of first fullerane C H ), the ratio Н/C is dehydrogenation of the synthesized samples of fulleranes 60 36 the same (1/12) as in fullerene С Н . However of so and quasi-fulleranes is started at 30-50 °C and the evacuation 60 60 steadfast interest of the researchers this controllable of hydrogen proceeds up to 700 °C. -

Functionalized Aromatics Aligned with the Three Cartesian Axes: Extension of Centropolyindane Chemistry*
Pure Appl. Chem., Vol. 78, No. 4, pp. 749–775, 2006. doi:10.1351/pac200678040749 © 2006 IUPAC Functionalized aromatics aligned with the three Cartesian axes: Extension of centropolyindane chemistry* Dietmar Kuck‡ Fakultät für Chemie, Universität Bielefeld, Universitätsstraße 25, D-33615 Bielefeld, Germany Abstract: The unique geometrical features and structural potential of the centropolyindanes, a complete family of novel, 3D polycyclic aromatic hydrocarbons, are discussed with respect to the inherent orthogonality of their arene units. Thus, the largest member of the family, centrohexaindane, a topologically nonplanar hydrocarbon, is presented as a “Cartesian hexa- benzene”, because each of its six benzene units is stretched into one of the six directions of the Cartesian space. This feature is discussed on the basis of the X-ray crystal structures of centrohexaindane and two lower members of the centropolyindane family, viz. the parent tribenzotriquinacenes. Recent progress in multiple functionalization and extension of the in- dane wings of selected centropolyindanes is reported, including several highly efficient six- and eight-fold C–C cross-coupling reactions. Some particular centropolyindane derivatives are presented, such as the first twelve-fold functionalized centrohexaindane and a tribenzo- triquinacene bearing three mutually orthogonal phenanthroline groupings at its molecular pe- riphery. Challenges to further extend the arene peripheries of the tribenzotriquinacenes and fenestrindanes to give, eventually, graphite cuttings bearing a central bowl- or saddle-shaped center are outlined, as is the hypothetical generation of a “giant” nanocube consisting of eight covalently bound tribenzotriquinacene units. Along these lines, our recent discovery of a re- lated, solid-state supramolecular cube, containing eight molecules of a particular tri- bromotrinitrotribenzotriquinacene of the same absolute configuration, is presented for the first time. -

Nuclear Magnetic Resonance Study of Cyclopentenone and Some of Its Derivatives Charles Edward Lyons Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1961 Nuclear magnetic resonance study of cyclopentenone and some of its derivatives Charles Edward Lyons Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Lyons, Charles Edward, "Nuclear magnetic resonance study of cyclopentenone and some of its derivatives " (1961). Retrospective Theses and Dissertations. 1975. https://lib.dr.iastate.edu/rtd/1975 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 62-1359 microfilmed exactly as received LYONS, Charles Edward, 1929- NUCLEAR MAGNETIC RESONANCE STUDY OF CYCLOPENTENONE AND SOME OF ITS DERIVA TIVES. Iowa State University of Science and Technology Ph.D., 1961 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan NUCISÂR MACBETIC RESONANCE STUDY OF CTCIJDPENTBNONE AHD SOIE OF ITS DERIVATIVES ty Charles Edward Iyons A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHHCSQPHT Major Subject! Organic Chemistry ApprovedJ Signature was redacted for privacy. Signature was redacted for privacy. Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa 1961 ii TABIE OF CONTENTS Page INTRODUCTION 1 HISTORICAL 2 DISCUSSION 12 SPECTRA 61 EXPERIMENTAL 91 SUMMAHT 96 ACKNOWIEDGEMBNTS 97 APPENDIX 98 1 INTRODUCTION During the past several years progress has been made in exploring the oheaLstiy of eyclopentenone and soma of its derivatives. -

SAFETY DATA SHEET Cyclopentane
SAFETY DATA SHEET Cyclopentane Section 1. Identification GHS product identifier : Cyclopentane Chemical name : cyclopentane Other means of : cyclopentane (dot); pentamethylene identification Product use : Synthetic/Analytical chemistry. Synonym : cyclopentane (dot); pentamethylene SDS # : 001124 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture AQUATIC HAZARD (ACUTE) - Category 3 AQUATIC HAZARD (LONG-TERM) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Highly flammable liquid and vapor. May form explosive mixtures in Air. Harmful to aquatic life with long lasting effects. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Avoid release to the environment. Response : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water or shower. Storage : Store in a well-ventilated place. Keep cool. Disposal : Dispose of contents and container in accordance with all local, regional, national and international regulations. Hazards not otherwise : None known. -
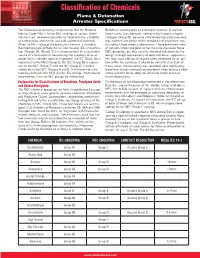
Classification of Chemicals
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

MSDS Cyclopentane (1).Pdf
MATERIAL SAFETY DATA SHEET (MSDS) CYCLOPENTANE 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION SUBSTANCE OR PREPATION TRADE NAME Cyclopentane CHEMICAL CLASSIFICATION Cycloparaffin, Naphthene COMPANY/ UNDERTAKING NAME AND Haldia Petrochemicals Limited, ADDRESS PO Box No 12, Haldia Plant PO Durgachak, Dist Midnapore West Bengal, India PIN 721 602 TELEPHONE 091-3224-274384 / 274400 EMERGENCY TELEPHONE NUMBER 091-3224-275916 2. COMPOSTION AND INFORMATION ON INGREDIENTS CHEMICAL CHEMICLAL CONTENT CAS EXPOSURE LIMITS IN AIR NAME FORMULA NUMBER (ppm) ACGIH ACGIH IDLH TLV- TLV- TWA STEL Cyclopentane C5H10 98.20 wt% 287-92-3 600 NA NA 2,2 Dimethyl (CH3)3CCH2CH3 0.28 wt% 75-83-2 500 1000 NA butane n- pentane n- C5H12 1.47 wt% 109-66-0 600 750 NA i- Pentane i- C5H12 0.05 wt% 78-78-4 600 750 NA Total Sulpher S < 1.0 wt. ppm 7704-34-9 NA NA NA 3. HAZARD CLASSIFICATION Highly flammable liquid and vapour. Vapour EMERGENCY OVERVIEW may cause flash fire. Harmful or Fatal is enter lungs and cause damage POTENTIAL HEALTH HAZARDS EYE SKIN INHALATION INGESTION OTHERS ACUTE To cause Not expected Dizziness, Abdominal prolonged or to be harmful headache, pain, significant to internal nausea, diarrhoea, eye irritation organs if Unconsciousness, dizziness, absorbed weakness nausea, sore through the throat skin. CHRONIC Repeated or prolonged contact with skin may cause dermatitis NFPA HAZARD HEALTH FLAMMABILITY REACTIVITY SPECIAL SIGNALS 2 3 0 - HAZCHEM CODE 3YE GHS-Classification Flammable liquids Category 2 Aspiration hazard Category 1 Chronic aquatic toxicity, Category 3 Acute aquatic toxicity, Category 3 Target Organ Systemic Toxicant - Single exposure, Category 3, Inhalation, Central nervous system Document Compiled By Approved By Issue No Rev. -
![The Synthesis of Hindered Aliphatic Ketones for the Future Production of Spiro [4.5] Decane Sesquiterpenes](https://docslib.b-cdn.net/cover/8996/the-synthesis-of-hindered-aliphatic-ketones-for-the-future-production-of-spiro-4-5-decane-sesquiterpenes-728996.webp)
The Synthesis of Hindered Aliphatic Ketones for the Future Production of Spiro [4.5] Decane Sesquiterpenes
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1986 The Synthesis of Hindered Aliphatic Ketones for the Future Production of Spiro [4.5] Decane Sesquiterpenes Stephen Lee Hodges College of William & Mary - Arts & Sciences Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Organic Chemistry Commons Recommended Citation Hodges, Stephen Lee, "The Synthesis of Hindered Aliphatic Ketones for the Future Production of Spiro [4.5] Decane Sesquiterpenes" (1986). Dissertations, Theses, and Masters Projects. Paper 1539625343. https://dx.doi.org/doi:10.21220/s2-jkzs-gq54 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. THE SYNTHESIS OF HINDERED ALIPHATIC KETONES FOR THE FUTURE PRODUCTION OF SPIRO[4.5] DECANE SESQUITERPENES A Thesis Presented to The Faculty of the Department of Chemistry The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Arts t>y Stephen Lee Hodges 1986 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Arts Author Approved, February 1986 David W. Thompson, Ph.D \\ j j D Trevor B. Hill, Ph.D. andolph A. Coleman, Ph.D. TABLE OF CONTENTS Page ABSTRACT................... vi INTRODUCTION........................................ 2 DISCUSSION............................................. 21 EXPERIMENTAL...........................................31 Spiro[4.5]dec-6-ene-lf 8-trione . .......... 31 2 -Isopropyl-l, 3-cyclopentanedione..............