Chapter 16: Ethers, Epoxides, and Sulfides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Chemistry
Wisebridge Learning Systems Organic Chemistry Reaction Mechanisms Pocket-Book WLS www.wisebridgelearning.com © 2006 J S Wetzel LEARNING STRATEGIES CONTENTS ● The key to building intuition is to develop the habit ALKANES of asking how each particular mechanism reflects Thermal Cracking - Pyrolysis . 1 general principles. Look for the concepts behind Combustion . 1 the chemistry to make organic chemistry more co- Free Radical Halogenation. 2 herent and rewarding. ALKENES Electrophilic Addition of HX to Alkenes . 3 ● Acid Catalyzed Hydration of Alkenes . 4 Exothermic reactions tend to follow pathways Electrophilic Addition of Halogens to Alkenes . 5 where like charges can separate or where un- Halohydrin Formation . 6 like charges can come together. When reading Free Radical Addition of HX to Alkenes . 7 organic chemistry mechanisms, keep the elec- Catalytic Hydrogenation of Alkenes. 8 tronegativities of the elements and their valence Oxidation of Alkenes to Vicinal Diols. 9 electron configurations always in your mind. Try Oxidative Cleavage of Alkenes . 10 to nterpret electron movement in terms of energy Ozonolysis of Alkenes . 10 Allylic Halogenation . 11 to make the reactions easier to understand and Oxymercuration-Demercuration . 13 remember. Hydroboration of Alkenes . 14 ALKYNES ● For MCAT preparation, pay special attention to Electrophilic Addition of HX to Alkynes . 15 Hydration of Alkynes. 15 reactions where the product hinges on regio- Free Radical Addition of HX to Alkynes . 16 and stereo-selectivity and reactions involving Electrophilic Halogenation of Alkynes. 16 resonant intermediates, which are special favor- Hydroboration of Alkynes . 17 ites of the test-writers. Catalytic Hydrogenation of Alkynes. 17 Reduction of Alkynes with Alkali Metal/Ammonia . 18 Formation and Use of Acetylide Anion Nucleophiles . -

Recent Advances in Titanium Radical Redox Catalysis
JOCSynopsis Cite This: J. Org. Chem. 2019, 84, 14369−14380 pubs.acs.org/joc Recent Advances in Titanium Radical Redox Catalysis Terry McCallum, Xiangyu Wu, and Song Lin* Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853, United States ABSTRACT: New catalytic strategies that leverage single-electron redox events have provided chemists with useful tools for solving synthetic problems. In this context, Ti offers opportunities that are complementary to late transition metals for reaction discovery. Following foundational work on epoxide reductive functionalization, recent methodological advances have significantly expanded the repertoire of Ti radical chemistry. This Synopsis summarizes recent developments in the burgeoning area of Ti radical catalysis with a focus on innovative catalytic strategies such as radical redox-relay and dual catalysis. 1. INTRODUCTION a green chemistry perspective, the abundance and low toxicity of Ti make its complexes highly attractive as reagents and Radical-based chemistry has long been a cornerstone of 5 1 catalysts in organic synthesis. synthetic organic chemistry. The high reactivity of organic IV/III radicals has made possible myriad new reactions that cannot be A classic example of Ti -mediated reactivity is the reductive ring opening of epoxides. This process preferentially readily achieved using two-electron chemistry. However, the − high reactivity of organic radicals is a double-edged sword, as cleaves and functionalizes the more substituted C O bond, the selectivity of these fleeting intermediates can be difficult to providing complementary regioselectivity to Lewis acid control in the presence of multiple chemotypes. In addition, promoted epoxide reactions. The synthetic value of Ti redox catalysis has been highlighted by their many uses in total catalyst-controlled regio- and stereoselective reactions involv- 6−10 ing free-radical intermediates remain limited,2 and the synthesis (Scheme 1). -

Unusual Regioselectivity in the Opening of Epoxides by Carboxylic Acid Enediolates
Molecules 2008, 13, 1303-1311 manuscripts; DOI: 10.3390/molecules13061303 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.org/molecules Communication Unusual Regioselectivity in the Opening of Epoxides by Carboxylic Acid Enediolates Luis R. Domingo, Salvador Gil, Margarita Parra* and José Segura Department of Organic Chemistry, Universitat de València, Dr. Moliner 50, 46100 Burjassot, Spain. Fax +34(9)63543831; E-mails: [email protected]; [email protected]; [email protected] * Author to whom correspondence should be addressed; E-mail: [email protected] Received: 29 May 2008; in revised form: 5 June 2008 / Accepted: 6 June 2008 / Published: 9 June 2008 Abstract: Addition of carboxylic acid dianions appears to be a potential alternative to the use of aluminium enolates for nucleophilic ring opening of epoxides. These conditions require the use of a sub-stoichiometric amount of amine (10% mol) for dianion generation and the previous activation of the epoxide with LiCl. Yields are good, with high regioselectivity, but the use of styrene oxide led, unexpectedly, to a mixture resulting from the attack on both the primary and secondary carbon atoms. Generally, a low diastereoselectivity is seen on attack at the primary center, however only one diastereoisomer was obtained from attack to the secondary carbon of the styrene oxide. Keywords: Lactones, lithium chloride, nucleophilic addition, regioselectivity, diastereoselectivity. Introduction Epoxides have been recognized among the most versatile compounds in organic synthesis, not only as final products [1] but as key intermediates for further manipulations. Accordingly, new synthetic developments are continuously being published [2]. Due to its high ring strain (around 27 Kcal/mol) its ring-opening, particularly with carbon-based nucleophiles, is a highly valuable synthetic strategy Molecules 2008, 13 1304 [3]. -

Highly Efficient Epoxidation of Olefins with Hydrogen Peroxide Oxidant Using Modified Silver Polyoxometalate Catalysts
African Journal of Pure and Applied Chemistry Vol. 7(2), pp. 50-55, February 2013 Available online at http://www.academicjournals.org/AJPAC DOI: 10.5897/AJPAC12.060 ISSN 1996 - 0840 ©2013 Academic Journals Full Length Research Paper Highly efficient epoxidation of olefins with hydrogen peroxide oxidant using modified silver polyoxometalate catalysts Emmanuel Tebandeke 1*, Henry Ssekaalo 1 and Ola F. Wendt 2 1Department of Chemistry, Makerere University, P. O. Box 7062 Kampala, Uganda. 2Organic Chemistry, Department of Chemistry, Lund University, P. O. Box 124, 221 00 Lund, Sweden. Accepted 31 January, 2013 The catalytic epoxidation of olefins is an important reaction in chemical industry since epoxides are versatile and important intermediates in the synthesis of fine chemicals and pharmaceuticals. The current epoxidation procedures often use stoichiometric organic and sometimes toxic inorganic oxidants that are less favourable from an environmental and economical point of view. In contrast to such processes, catalytic epoxidation processes employing H2O2 oxidant are preferred for green processing. Herein is reported a highly efficient green process for the epoxidation of olefins using H2O2 oxidant and modified silver polyoxometalate catalysts at 65°C. The preparation and activity of the catalysts are described. The method enjoys >90% conversion and ≥99% selectivity to the epoxide for a variety of cyclic and linear olefins including terminal ones. The catalysts are easily recovered by filtration and are reusable several times. Key words: Epoxidation, olefins, hydrogen peroxide, polyoxometalate, silver catalysts. INTRODUCTION The catalytic epoxidation of olefins is very important in compared to organic peroxides and peracids (Jones, the chemical industry since epoxides are versatile and 1999; Lane and Burgess, 2003). -

Part I. the Total Synthesis Of
AN ABSTRACT OF THE THESIS OF Lester Percy Joseph Burton forthe degree of Doctor of Philosophy in Chemistry presentedon March 20, 1981. Title: Part 1 - The Total Synthesis of (±)-Cinnamodialand Related Drimane Sesquiterpenes Part 2 - Photochemical Activation ofthe Carboxyl Group Via NAcy1-2-thionothiazolidines Abstract approved: Redacted for privacy DT. James D. White Part I A total synthesis of the insect antifeedant(±)-cinnamodial ( ) and of the related drimanesesquiterpenes (±)-isodrimenin (67) and (±)-fragrolide (72)are described from the diene diester 49. Hydro- boration of 49 provided the C-6oxygenation and the trans ring junction in the form of alcohol 61. To confirm the stereoselectivity of the hydroboration, 61 was convertedto both (t)-isodrimenin (67) and (±)-fragrolide (72) in 3 steps. A diisobutylaluminum hydride reduction of 61 followed by a pyridiniumchlorochromate oxidation and treatment with lead tetraacetate yielded the dihydrodiacetoxyfuran102. The base induced elimination of acetic acid preceded theepoxidation and provided 106 which contains the desired hydroxy dialdehydefunctionality of cinnamodial in a protected form. The epoxide 106 was opened with methanol to yield the acetal 112. Reduction, hydrolysis and acetylation of 112 yielded (t)- cinnamodial in 19% overall yield. Part II - Various N- acyl- 2- thionothiazolidineswere prepared and photo- lysed in the presence of ethanol to provide the corresponding ethyl esters. The photochemical activation of the carboxyl function via these derivatives appears, for practical purposes, to be restricted tocases where a-keto hydrogen abstraction and subsequent ketene formation is favored by acyl substitution. Part 1 The Total Synthesis of (±)-Cinnamodial and Related Drimane Sesquiterpenes. Part 2 Photochemical Activation of the Carboxyl Group via N-Acy1-2-thionothiazolidines. -

Epoxidation of Olefins Using Molecular Oxygen
Epoxidation of Olefins Using Molecular Oxygen Zhongxing Huang Sep 16th, 2015 1 Major Challenge in Catalysis Key points . Low temperature . Selective May 31st, 1993, C&EN News. 2 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product Iron-Containing Enzymes, RSC Publishing, 2011. 3 Most Ancient Chemistry-Oxygenase Dioxygenase incorporates both oxygen atoms into the product Shen, B.; Gould, S. J. Biochemistry 1991, 30, 8936. Gould, S. J.; Kirchmeier, M. J.; LaFever, R. E. J. Am. Chem. Soc. 1996, 118, 7663. 4 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product . Ideal system should avoid use of reductants Dioxygenase incorporates both oxygen atoms into the product 5 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product . Ideal system should avoid use of reductants Dioxygenase incorporates both oxygen atoms into the product Ideal Dioxygenase incorporates both oxygen atoms into the epoxide 6 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product . Ideal system should avoid use of reductants Dioxygenase incorporates both oxygen atoms into the product Ideal Dioxygenase incorporates both oxygen atoms into the epoxide 7 Industrial Process- EO and PO . Top chemicals produced in US (2004,103 ton) 1 sulfuric acid 35954 2 nitrogen 30543 3 ethylene 25682 4 oxygen 25568 5 propylene 15345 6 chlorine 12166 7 ethylene dichloride 12163 8 phosphoric acid 11463 9 ammonia 10762 10 sodium hydroxide 9508 11 benzene 7675 12 nitric acid 6703 13 ammonium nitrate 6021 14 ethylbenzene 5779 15 urea 5755 16 styrene 5394 17 hydrochloric acid 5012 18 ethylene oxide 3772 19 cumene 3736 20 ammonium sulfate 2643 8 Industrial Process- EO and PO . -

Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers
Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers • Ethers (R–O–R’): – Organic derivatives of water, having two organic groups bonded to the same oxygen atom © 2016 Cengage Learning 2 NAMES AND PROPERTIES OF ETHERS 3 Nomenclature: Common Names • Simple ethers are named by identifying two organic substituents and adding the word ether – Name the groups in alphabetical order – Symmetrical: Use dialkyl or just alkyl © 2016 Cengage Learning 4 Nomenclature: IUPAC Names • The more complex alkyl group is the parent name • The group with the oxygen becomes an alkoxy group © 2016 Cengage Learning 5 Nomenclature: Cyclic Ethers (Heterocycles) • Heterocyclic: Oxygen is part of the ring. O • Epoxides (oxiranes) H2C CH2 O • Oxetanes • Furans (Oxolanes) O O • Pyrans (Oxanes) O O O • Dioxanes O © 2013 Pearson Education, Inc. 6 Epoxide Nomenclature • Name the starting alkene and add “oxide” © 2013 Pearson Education, Inc. 7 Epoxide Nomenclature • The oxygen can be treated as a substituent (epoxy) on the compound • Use numbers to specify position • Oxygen is 1, the carbons are 2 and 3 • Substituents are named in alphabetical order © 2013 Pearson Education, Inc. 8 Properties of Ethers • Possess nearly the same geometry as water – Oxygen atom is sp3-hybridized – Bond angles of R–O–R bonds are approximately tetrahedral • Polar C—O bonds © 2013 Pearson Education, Inc. 9 Properties of Ethers: Hydrogen Bond • Hydrogen bond is a attractive interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom • Ethers cannot hydrogen bond with other ether molecules, so they have a lower boiling point than alcohols • Ether molecules can hydrogen bond with water and alcohol molecules • They are hydrogen bond acceptors © 2013 Pearson Education, Inc. -

20 More About Oxidation–Reduction Reactions
More About 20 Oxidation–Reduction Reactions OOC n important group of organic reactions consists of those that O A involve the transfer of electrons C from one molecule to another. Organic chemists H OH use these reactions—called oxidation–reduction reactions or redox reactions—to synthesize a large O variety of compounds. Redox reactions are also important C in biological systems because many of these reactions produce HH energy. You have seen a number of oxidation and reduction reactions in other chapters, but discussing them as a group will give you the opportunity to CH3OH compare them. In an oxidation–reduction reaction, one compound loses electrons and one com- pound gains electrons. The compound that loses electrons is oxidized, and the one that gains electrons is reduced. One way to remember the difference between oxidation and reduction is with the phrase “LEO the lion says GER”: Loss of Electrons is Oxi- dation; Gain of Electrons is Reduction. The following is an example of an oxidation–reduction reaction involving inorganic reagents: Cu+ + Fe3+ ¡ Cu2+ + Fe2+ In this reaction,Cu+ loses an electron, so Cu+ is oxidized. Fe3+ gains an electron, so Fe3+ is reduced. The reaction demonstrates two important points about oxidation– reduction reactions. First, oxidation is always coupled with reduction. In other words, a compound cannot gain electrons (be reduced) unless another compound in the reaction simultaneously loses electrons (is oxidized). Second, the compound that is oxidized (Cu+) is called the reducing agent because it loses the electrons that are used to reduce the other compound (Fe3+). Similarly, the compound that is reduced (Fe3+) is called the oxidizing agent because it gains the electrons given up by the other compound (Cu+) when it is oxidized. -

Reactions of Epoxides with Neighbouring Nucleophiles
REACTIONS OF EPOXIDES WITH NEIGHBOURING NUCLEOPHILES A thesis presented for the degree of Doctor o.f Philosophy in Chemistry in the University of Canterbury, Christchurch, New Zealand. by W.H. Swallow 1972 CONTENTS ABSTRACT INTRODUCTION 1 Acid catalysed reactions of epoxides 1 (i) Mechanism of acid catalysed reactions of epoxides with nucleophiles 4 (ii) Ring formation by acid catalysed intramolecular nucleophilic attack of epoxides 10 (iii) Mechanism of acid catalysed rearrangements of epoxides to give carbonyl compounds. 14 Objectives and work undertaken 26 NEIGHBOURING HYDROXYL GROUPS 28 Preparation of the epoxyalcohols 28 The acid catalysed reactions of the epoxyalcohols 36 Rearrangements of epoxyheptanols (26,32) and epoxy- hexanols (24,30) 37 Mechanism of rearrangements of epoxyheptanols (26,32} and epoxyhexanols (24,30) 40 Rearrangements of epoxypentanols (22,28} 42 Mechanism of rearrangements of epoxypentanols (22,28) 44 THE ACID CATALYSED REARRANGEMENTS OF EPOXIDES WITH NEIGHBOURING ACETATE GROUPS 53 Introduction 53 Mechanisms of the acid catalysed reactions of epoxides with neighbouring ester groups 54 The acid catalysed reactions of epoxyacetates 59 Rearrangements of epoxypentanol acetates {23,29) 60 Rearrangements of epoxyhexanol acetates (25,31} 61 Rearrangements of epoxyheptanol acetates (27,33) 64 18 Rearrangements of o enriched epoxyacetates 65 Mechanism of formation of tetrahydrofuranol derivatives from epoxyacetates 66 Mechanism of formation of fluorohydrins and carbonyl compounds from epoxyacetates 73 EXPERIMENTAL 76 REFERENCES 109 ABSTRACT The syntheses and acid catalysed rearrangements of cis- and trans-3,4-epoxypentan-1-ols (28,22), 4,5-epoxyhexan-1-ols (30,24) and 5,6-epoxyheptan-1-ols (32,26) have been described. Isomerizations of these compounds gave cyclic ether derivatives as the major products. -

Advices for Studying Organic Chemistry
Table of Contents Partial table of contents: Carbon Compounds and Chemical Bonds. Representative Carbon Compounds. An Introduction to Organic Reactions: Acids and Bases. Alkanes and Cycloalkanes: Conformations of Molecules. Stereochemistry: Chiral Molecules. Alkenes and Alkynes I: Properties and Synthesis. Alkenes and Alkynes II: Addition Reactions. Radical Reactions. Alcohols and Ethers. Conjugated Unsaturated Systems. Aromatic Compounds. Reactions of Aromatic Compounds. Aldehydes and Ketones I: Nucleophilic Additions to the Carbonyl Group. Aldehydes and Ketones II: Aldol Reactions. Carboxylic Acids and Their Derivatives: Nucleophilic Substitution at the Acyl Carbon. Amines. Carbohydrates. Lipids. Answers to Selected Problems. Glossary. Index. Solomons/Advices ADVICES FOR STUDYING ORGANIC CHEMISTRY 1. Keep up with your studying day to day –– never let yourself get behind, or better yet, be a little ahead of your instructor. Organic chemistry is a course in which one idea almost always builds on another that has gone before. 2. Study materials in small units, and be sure that you understand each new section before you go on to the next. Because of the cumulative nature of organic chemistry, your studying will be much more effective if you take each new idea as it comes and try to understand it completely before you move onto the nest concept. 3. Work all of the in-chapter and assigned problems. 4. Write when you study. Write the reactions, mechanisms, structures, and so on, over and over again. You need to know the material so thoroughly that you can explain it to someone else. This level of understanding comes to most of us (those of us without photographic memories) through writing. -

Synthesis and Polymerization of Several Ester Substituted Epoxides. John, Muggee University of Massachusetts Amherst
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations 1896 - February 2014 1-1-1982 Synthesis and polymerization of several ester substituted epoxides. John, Muggee University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_1 Recommended Citation Muggee, John,, "Synthesis and polymerization of several ester substituted epoxides." (1982). Doctoral Dissertations 1896 - February 2014. 670. https://scholarworks.umass.edu/dissertations_1/670 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations 1896 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. SYNTHESIS AND POLYMERIZATION OF SEVERAL ESTER SUBSTITUTED EPOXIDES A Dissertation Presented By JOHN MUGGEE Submitted to the Graduate School of the University of Massachusetts in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Pebtuary 1982. Polymer Science and Engineering SYNTHESIS AND POLYMERIZATION OF SEVERAL ESTER SUBSTITUTED EPOXIDES A Dissertation Presented By JOHN MUGGEE Approved as to style and content by: Otto Vogl,/ Chairpd^rson of Committee R. S. Stein, Member Polymer Science and Engineering 11( JOHN MUGGEE All rights reserved . ACKNOWLEDGMENTS The author would like to offer his deepest thanks and gratitude to his parents for their love and guidance thoughout his life, and to Professor Otto Vogl for his friendship and professional guidance during the course of this research. The efforts and helpful suggestions con- tributed by Professors Richard S. Stein and James C. W. Chien are also appreciated. The author would also like to thank his laboratory coworkers for making this an interest ing and productive period of his life. -
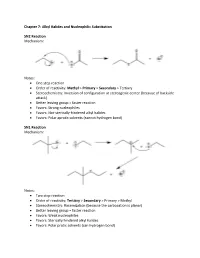
Alkyl Halides and Nucleophilic Substitution SN2 Reaction
Chapter 7: Alkyl Halides and Nucleophilic Substitution SN2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Methyl > Primary > Secondary > Tertiary • Stereochemistry: Inversion of configuration at stereogenic center (because of backside attack) • Better leaving group = faster reaction • Favors: Strong nucleophiles • Favors: Not-sterically-hindered alkyl halides • Favors: Polar aprotic solvents (cannot hydrogen bond) SN1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary > Methyl • Stereochemistry: Racemization (because the carbocation is planar) • Better leaving group = faster reaction • Favors: Weak nucleophiles • Favors: Sterically hindered alkyl halides • Favors: Polar protic solvents (can hydrogen bond) Important Trends Chapter 8: Alkyl Halides and Elimination Reactions E2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: antiperiplanar arrangement of H and X • Better leaving group = faster reaction • Favors: Polar aprotic solvents, strong bases • Products follow Zaitsev rule (more substituted alkene is the major product) E1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: Trigonal planar carbocation intermediate • Better leaving group = faster reaction • Favors: Polar protic solvents, weak bases • Products follow Zaitsev rule Chapter 9: Alcohols, Ethers, and Epoxides Preparation of Alcohols Mechanism: Notes: • SN2 mechanism