Neurocranial Histomorphometrics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Frontosphenoidal Synostosis: a Rare Cause of Unilateral Anterior Plagiocephaly
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Childs Nerv Syst (2007) 23:1431–1438 DOI 10.1007/s00381-007-0469-4 ORIGINAL PAPER Frontosphenoidal synostosis: a rare cause of unilateral anterior plagiocephaly Sandrine de Ribaupierre & Alain Czorny & Brigitte Pittet & Bertrand Jacques & Benedict Rilliet Received: 30 March 2007 /Published online: 22 September 2007 # Springer-Verlag 2007 Abstract Conclusion Frontosphenoidal synostosis must be searched Introduction When a child walks in the clinic with a in the absence of a coronal synostosis in a child with unilateral frontal flattening, it is usually associated in our anterior unilateral plagiocephaly, and treated surgically. minds with unilateral coronal synostosis. While the latter might be the most common cause of anterior plagiocephaly, Keywords Craniosynostosis . Pediatric neurosurgery. it is not the only one. A patent coronal suture will force us Anterior plagiocephaly to consider other etiologies, such as deformational plagio- cephaly, or synostosis of another suture. To understand the mechanisms underlying this malformation, the development Introduction and growth of the skull base must be considered. Materials and methods There have been few reports in the Harmonious cranial growth is dependent on patent sutures, literature of isolated frontosphenoidal suture fusion, and and any craniosynostosis might lead to an asymmetrical we would like to report a series of five cases, as the shape of the skull. The anterior skull base is formed of recognition of this entity is important for its treatment. different bones, connected by sutures, fusing at different ages. The frontosphenoidal suture extends from the end of Presented at the Consensus Conference on Pediatric Neurosurgery, the frontoparietal suture, anteriorly and inferiorly in the Rome, 1–2 December 2006. -
Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

Single‑Staged Resections and 3D Reconstructions of the Nasion, Glabella, Medial Orbital Wall, and Frontal Sinus and Bone
OPEN ACCESS Editor: James I. Ausman, MD, PhD For entire Editorial Board visit : University of California, Los http://www.surgicalneurologyint.com Angeles, CA, USA SNI: Skull Base, a supplement to Surgical Neurology International Case Report Single‑staged resections and 3D reconstructions of the nasion, glabella, medial orbital wall, and frontal sinus and bone: Long‑term outcome and review of the literature Jeremy Ciporen, Brandon P. Lucke‑Wold1, Gustavo Mendez2, Anton Chen3, Amit Banerjee4, Paul T. Akins4, Ben J. Balough3 Department of Neurological Surgery, and 2Department of Diagnostic Radiology, Oregon Health and Science University, Portland, Oregon, 1Department of Neurosurgery, West Virginia University, Morgantown, West Virginia, 3Departments of ENT, and 4Neurosurgery, Kaiser Permanente, Sacramento, California, USA E‑mail: *Jeremy Ciporen ‑ [email protected]; Brandon P. Lucke‑Wold ‑ [email protected]; Gustavo Mendez ‑ [email protected]; Anton Chen ‑ [email protected]; Amit Banerjee ‑ [email protected]; Paul T. Akins ‑ [email protected]; Ben J. Balough ‑ [email protected] *Corresponding author Received: 11 March 16 Accepted: 10 September 16 Published: 26 December 16 Abstract Background: Aesthetic facial appearance following neurosurgical ablation of frontal fossa tumors is a primary concern for patients and neurosurgeons alike. Craniofacial reconstruction procedures have drastically evolved since the development of three‑dimensional computed tomography imaging and computer‑assisted programming. Traditionally, two‑stage approaches for resection and reconstruction were used; however, these two‑stage approaches have many complications including cerebrospinal fluid leaks, necrosis, and pneumocephalus. Case Description: We present two successful cases of single‑stage osteoma resection and craniofacial reconstruction in a 26‑year‑old female and 65‑year‑old male. -

Anatomy of the Periorbital Region Review Article Anatomia Da Região Periorbital
RevSurgicalV5N3Inglês_RevistaSurgical&CosmeticDermatol 21/01/14 17:54 Página 245 245 Anatomy of the periorbital region Review article Anatomia da região periorbital Authors: Eliandre Costa Palermo1 ABSTRACT A careful study of the anatomy of the orbit is very important for dermatologists, even for those who do not perform major surgical procedures. This is due to the high complexity of the structures involved in the dermatological procedures performed in this region. A 1 Dermatologist Physician, Lato sensu post- detailed knowledge of facial anatomy is what differentiates a qualified professional— graduate diploma in Dermatologic Surgery from the Faculdade de Medician whether in performing minimally invasive procedures (such as botulinum toxin and der- do ABC - Santo André (SP), Brazil mal fillings) or in conducting excisions of skin lesions—thereby avoiding complications and ensuring the best results, both aesthetically and correctively. The present review article focuses on the anatomy of the orbit and palpebral region and on the important structures related to the execution of dermatological procedures. Keywords: eyelids; anatomy; skin. RESU MO Um estudo cuidadoso da anatomia da órbita é muito importante para os dermatologistas, mesmo para os que não realizam grandes procedimentos cirúrgicos, devido à elevada complexidade de estruturas envolvidas nos procedimentos dermatológicos realizados nesta região. O conhecimento detalhado da anatomia facial é o que diferencia o profissional qualificado, seja na realização de procedimentos mini- mamente invasivos, como toxina botulínica e preenchimentos, seja nas exéreses de lesões dermatoló- Correspondence: Dr. Eliandre Costa Palermo gicas, evitando complicações e assegurando os melhores resultados, tanto estéticos quanto corretivos. Av. São Gualter, 615 Trataremos neste artigo da revisão da anatomia da região órbito-palpebral e das estruturas importan- Cep: 05455 000 Alto de Pinheiros—São tes correlacionadas à realização dos procedimentos dermatológicos. -
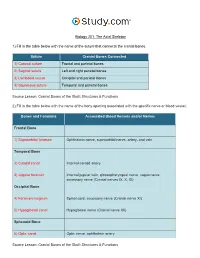
The Axial Skeleton Visual Worksheet
Biology 201: The Axial Skeleton 1) Fill in the table below with the name of the suture that connects the cranial bones. Suture Cranial Bones Connected 1) Coronal suture Frontal and parietal bones 2) Sagittal suture Left and right parietal bones 3) Lambdoid suture Occipital and parietal bones 4) Squamous suture Temporal and parietal bones Source Lesson: Cranial Bones of the Skull: Structures & Functions 2) Fill in the table below with the name of the bony opening associated with the specific nerve or blood vessel. Bones and Foramina Associated Blood Vessels and/or Nerves Frontal Bone 1) Supraorbital foramen Ophthalmic nerve, supraorbital nerve, artery, and vein Temporal Bone 2) Carotid canal Internal carotid artery 3) Jugular foramen Internal jugular vein, glossopharyngeal nerve, vagus nerve, accessory nerve (Cranial nerves IX, X, XI) Occipital Bone 4) Foramen magnum Spinal cord, accessory nerve (Cranial nerve XI) 5) Hypoglossal canal Hypoglossal nerve (Cranial nerve XII) Sphenoid Bone 6) Optic canal Optic nerve, ophthalmic artery Source Lesson: Cranial Bones of the Skull: Structures & Functions 3) Label the anterior view of the skull below with its correct feature. Frontal bone Palatine bone Ethmoid bone Nasal septum: Perpendicular plate of ethmoid bone Sphenoid bone Inferior orbital fissure Inferior nasal concha Maxilla Orbit Vomer bone Supraorbital margin Alveolar process of maxilla Middle nasal concha Inferior nasal concha Coronal suture Mandible Glabella Mental foramen Nasal bone Parietal bone Supraorbital foramen Orbital canal Temporal bone Lacrimal bone Orbit Alveolar process of mandible Superior orbital fissure Zygomatic bone Infraorbital foramen Source Lesson: Facial Bones of the Skull: Structures & Functions 4) Label the right lateral view of the skull below with its correct feature. -

Inter-Parietal Bones in Neurocranium of Human Adult Dry Skulls K
International Journal of Current Medical And Applied Sciences, 2015, December, 9(1),44-47. ORIGINAL RESEARCH ARTICLE INCA- Inter-Parietal Bones in Neurocranium of Human Adult Dry Skulls K. Arumugam 1 & A. Arunkumar 2 1Assistant Professor, Department of Anatomy, Tirunelveli Medical College, Tirunelveli.Tamil Nadu, India. 2Tutor, Andamon Nicobar Island of Institute of Medical Sciences, Andaman. India. ---------------------------------------------------------------------------------------------------------------------------------------------------- Abstract: Skull means the skeleton of the head including mandible and calvaria means skull after the bones of the face have been removed. In this the word skull is frequently used instead of cranium as a matter of convenience and common usage Objectives: To determine the incidence and type of INCA variants in human adult dry skull. Materials and Methods In this study, 100 human dried skulls were analyzed. All the skulls were taken from the Institute of Anatomy, Madras Medical College, Chennai. Results: Gross incidence of INCA was found to be 2%. The INCA occurred in single. Conclusion: This study gives idea of INCA regarding gross incidence, number and type. This knowledge is useful for neurosurgeons, anthropologists and radiologists. Key words: Neurocranium, INCA bone, Sutural bones. Introduction: According to the catalogues of craniological collections, fontanelle and paired posterior-lateral fontanelle. skull means the skeleton of the head including These fontanelles allows the expansion of the foetal mandible and calvaria means skull after the bones of head to accommodate the rapid enlargement of brain the face have been removed. In this the word skull is which take place during postnatal life. During frequently used instead of cranium as a matter of parturition the fontanelles also help in moulding of the convenience and common usage [2]. -

Compact Bone Spongy Bone
Spongy bone Compact bone © 2018 Pearson Education, Inc. 1 (b) Flat bone (sternum) (a) Long bone (humerus) (d) Irregular bone (vertebra), right lateral view (c) Short bone (talus) © 2018 Pearson Education, Inc. 2 Articular cartilage Proximal epiphysis Spongy bone Epiphyseal line Periosteum Compact bone Medullary cavity (lined by endosteum) Diaphysis Distal epiphysis (a) © 2018 Pearson Education, Inc. 3 Trabeculae of spongy bone Osteon (Haversian Perforating system) (Volkmann’s) canal Blood vessel continues into medullary cavity containing marrow Blood vessel Lamellae Compact bone Central (Haversian) canal Perforating (Sharpey’s) fibers Periosteum Periosteal blood vessel (a) © 2018 Pearson Education, Inc. 4 Lamella Osteocyte Canaliculus Lacuna Central Bone matrix (Haversian) canal (b) © 2018 Pearson Education, Inc. 5 Osteon Interstitial lamellae Lacuna Central (Haversian) canal (c) © 2018 Pearson Education, Inc. 6 Articular cartilage Hyaline Spongy cartilage bone New center of bone growth New bone Epiphyseal forming plate cartilage Growth Medullary in bone cavity width Bone starting Invading to replace Growth blood cartilage in bone vessels length New bone Bone collar forming Hyaline Epiphyseal cartilage plate cartilage model In an embryo In a fetus In a child © 2018 Pearson Education, Inc. 7 Bone growth Bone grows in length because: Articular cartilage 1 Cartilage grows here. Epiphyseal plate 2 Cartilage is replaced by bone here. 3 Cartilage grows here. © 2018 Pearson Education, Inc. 8 Bone remodeling Growing shaft is remodeled as: Articular cartilage Epiphyseal plate 1 Bone is resorbed by osteoclasts here. 2 Bone is added (appositional growth) by osteoblasts here. 3 Bone is resorbed by osteoclasts here. © 2018 Pearson Education, Inc. 9 Hematoma External Bony callus callus of spongy bone New Internal blood callus vessels Healed (fibrous fracture tissue and Spongy cartilage) bone trabecula 1 Hematoma 2 Fibrocartilage 3 Bony callus 4 Bone remodeling forms. -

The Frontal Bone As a Proxy for Sex Estimation in Humans: a Geometric
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2014 The frontal bone as a proxy for sex estimation in humans: a geometric morphometric analysis Lucy Ann Edwards Hochstein Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Social and Behavioral Sciences Commons Recommended Citation Hochstein, Lucy Ann Edwards, "The frontal bone as a proxy for sex estimation in humans: a geometric morphometric analysis" (2014). LSU Master's Theses. 1749. https://digitalcommons.lsu.edu/gradschool_theses/1749 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. THE FRONTAL BONE AS A PROXY FOR SEX ESTIMATION IN HUMANS: A GEOMETRIC MORPHOMETRIC ANALYSIS A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Anthropology in The Department of Geography and Anthropology By Lucy A. E. Hochstein B.A., George Mason University, 2009 May 2014 ACKNOWLEDGEMENTS Completing a master’s thesis was the most terrifying aspect of graduate school and I must acknowledge the people and pets that helped me on this adventure. I could not have asked for a better committee chair than Dr. Ginny Listi, who stuck by me when everything fell apart and was always willing to offer support and help me find solutions. -

A Heads up on Craniosynostosis
Volume 1, Issue 1 A HEADS UP ON CRANIOSYNOSTOSIS Andrew Reisner, M.D., William R. Boydston, M.D., Ph.D., Barun Brahma, M.D., Joshua Chern, M.D., Ph.D., David Wrubel, M.D. Craniosynostosis, an early closure of the growth plates of the skull, results in a skull deformity and may result in neurologic compromise. Craniosynostosis is surprisingly common, occurring in one in 2,100 children. It may occur as an isolated abnormality, as a part of a syndrome or secondary to a systemic disorder. Typically, premature closure of a suture results in a characteristic cranial deformity easily recognized by a trained observer. it is usually the misshapen head that brings the child to receive medical attention and mandates treatment. This issue of Neuro Update will present the diagnostic features of the common types of craniosynostosis to facilitate recognition by the primary care provider. The distinguishing features of other conditions commonly confused with craniosynostosis, such as plagiocephaly, benign subdural hygromas of infancy and microcephaly, will be discussed. Treatment options for craniosynostosis will be reserved for a later issue of Neuro Update. Types of Craniosynostosis Sagittal synostosis The sagittal suture runs from the anterior to the posterior fontanella (Figure 1). Like the other sutures, it allows the cranial bones to overlap during birth, facilitating delivery. Subsequently, this suture is the separation between the parietal bones, allowing lateral growth of the midportion of the skull. Early closure of the sagittal suture restricts lateral growth and creates a head that is narrow from ear to ear. However, a single suture synostosis causes deformity of the entire calvarium. -

(Frontal Sinus, Coronal Suture) Parietal Bone
Axial Skeleton Skull (cranium) Frontal Bone (frontal sinus, coronal suture) Parietal Bone (sagittal suture) Sphenoid Bone (sella turcica, sphenoid sinus) Temporal Bone (mastoid process, styloid process, external auditory meatus) malleus incus stapes Occipital Bone (occipital condyles, foramen magnum) Ethmoid Bone (nasal conchae, cribriform plate, crista galli, ethmoid sinus, perpendicular plate) Lacrimal Bone Zygomatic Bone Maxilla Bone (hard palate, palatine process, maxillary sinus) Palatine Bone Nasal Bone Vomer Bone Mandible Hyoid Bone Vertebral Column (general markings: body, vertebral foramen, transverse process, spinous process, superior and inferior articular processes) Cervical Vertebrae (transverse foramina) Atlas (absence of body, "yes" movement) Axis (dens, "no" movement) Thoracic Vertebrae (facets on body and transverse processes) Lumbar Vertebrae (largest) Sacral Vertebrae 5 fused vertebrae) Coccyx (3 to 5 vestigial vertebrae, body only on most) Bony Thorax Ribs (costal cartilage, true ribs, false ribs, floating ribs, facets) Sternum Manubrium Body Xiphoid Process Appendicular Skeleton Upper Limb Pectoral Girdle Scapula (acromion, coracoid process, glenoid cavity, spine) Clavicle Upper Arm Humerus (head, greater tubercle, lesser tubercle, olecranon fossa) Forearm Radius Ulna (olecranon process) Hand Carpals Metacarpals Phalanges Lower Limb Pelvic Girdle Os Coxae (sacroiliac joint, acetabulum, obturator foramen, false pelvis, difference between male and female pelvis) Ilium (iliac crest) Ischium (ischial tuberosity) Pubis (pubic symphysis) Thigh Femur (head, neck) Patella Lower Leg Tibia Fibula Foot Tarsals Metatarsals Phalanges. -

Bone-Axial Skeleton
BIO 176: Human Anatomy Lab Bone Practical: Lecture Bone-Axial Skeleton Speaker: Heidi Peterson What you will see in the following presentation are all of the bones and features and markings you will need to know for your upcoming practical. We are going to start with the skull. On the skull you will see an anterior view, which is like looking at somebody face to face. On the anterior view the use of your regional terms is going to be necessary. You learned them for a reason and they are going to help you with bones. The first bone you are going to see is your forehead, but it is actually called the frontal bone. The next bone you see is the nasal bone. It also forms the bridge of your nose. You might know it as the cheek bone, but anatomically correct it is called the zygomatic. If you feel in between your two nostrils it might seem strange, but you are going to find a bony protuberance that is called the vomer. The vomer is the bone that gives shape to your lip. That little frenulum comes from the vomer. Also in the skull you will find two big jaw bones. The top jaw bone is the Maxillae. And the bottom jaw bone is the Mandible. There will be features on each of these bones but we will talk about those in just a bit. Starting with the markings or features you will see a circle around something above the orbit of your eye. Anything that is above something anatomically is called superior or supra.