University of London Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1.1 10 Oxidation of Alcohols and Aldehydes
1.1 10 Oxidation of alcohols and aldehydes By the end of this spread, you should be able to … 1Describe the oxidation of primary alcohols to form aldehydes and carboxylic acids. 1Describe the oxidation of secondary alcohols to form ketones. 1Describe the oxidation of aldehydes to form carboxylic acids. Key definition Oxidation of alcohols You will recall from your AS chemistry studies that primary and secondary alcohols can A redox reaction is one in which both reduction and oxidation take place. be oxidised using an oxidising agent. s !SUITABLEOXIDISINGAGENTISASOLUTIONCONTAININGACIDIlEDDICHROMATEIONS + 2− H /Cr2O7 . s 4HEOXIDISINGMIXTURECANBEMADEFROMPOTASSIUMDICHROMATE +2Cr2O7, and sulfuric acid, H2SO. During the reaction, the acidified potassium dichromate changes from orange to green. Remember that tertiary alcohols are not oxidised by acidified dichromate ions. Primary alcohols Primary alcohols can be oxidised to aldehydes and to carboxylic acids (see AS Chemistry spread 2.2.3). H H H O H O Oxidation Oxidation H CCOH H CC H CC H H H H H OH Primary alcohol Aldehyde Carboxylic acid Figure 1 Ethanol oxidised to ethanal, and finally to ethanoic acid The equation for the oxidation of ethanol to the aldehyde ethanal is shown below. Note that the oxidising agent is shown as [O] – this simplifies the equation. CH3CH2OH + [O] }m CH3CHO + H2O When preparing aldehydes in the laboratory, you will need to distil the aldehyde from the reaction mixture as it is formed. This prevents the aldehyde from being oxidised further to a carboxylic acid. Key definition When making the carboxylic acid, the reaction mixture is usually heated under reflux before distilling the product off. -

Enhanced Butanol Production by Free and Immobilized Clostridium Sp
Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells Using Butyric Acid as Co-Substrate Laili Gholizadeh This thesis comprises 30 ECTS credits and is a compulsory part in the Master of Science with a Major in Chemical Engineering – Applied Biotechnology 120 ECTS credits No. 10/2009 Title: Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells using Butyric Acid as Co-Substrate. Author: Laili Gholizadeh Baroghi (e-mail: [email protected]) Master Thesis Subject Category: Biotechnology (Bioprocess Engineering – Biofuels) University College of Borås School of Engineering SE-501 90 BORÅS Telephone: (+46) 033 435 4640 Examiner: Prof. Mohammad Taherzadeh Supervisor and Thesis Advisor: Prof. Shang–Tian Yang Supervisor Address: OSU–Ohio State University 125 Koffolt Laboratories 140 West 19th Ave. Columbus, OH 43210–1185, USA Client: Ohio State University (OSU), Chemical & Biomolecular Engineering Department Prof. Shang–Tian Yang Columbus, Ohio; USA. Date: 08–12–2009 Keywords: Bio-butanol y Acetone–Butanol–Ethanol (ABE) y ABE- fermentation y Butyric acid y Clostridium y C. acetobutylicum ATCC 824 y C. beijerinckii ATCC 55025 y C. beijerinckii BA 101 y C. beijerinckii NCIMB 8052 y Fibrous-bed Bioreactor (FBB) y Batch y Suspended cell culture y Immobilized cell system. DEDICATION I would like to dedicate this M.Sc. Thesis to my beloved Family for all their love and encouragement and for always been supportive of my choices. “I am among those who think that science has great beauty. A scientist in his laboratory is not only a technician: he is also a child placed before natural phenomena, which impress him like a fairy tale.” − Marie Curie ABSTRACT Butanol production by four different Clostridium sp. -
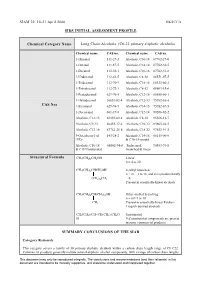
Long Chain Alcohols (C6-22 Primary Aliphatic Alcohols)
SIAM 22, 18-21 April 2006 UK/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Long Chain Alcohols (C6-22 primary aliphatic alcohols) Chemical name CAS no. Chemical name CAS no. 1-Hexanol 111-27-3 Alcohols, C16-18 67762-27-0 1-Octanol 111-87-5 Alcohols, C14-18 67762-30-5 1-Decanol 112-30-1 Alcohols, C10-16 67762-41-8 1-Undecanol 112-42-5 Alcohols, C8-18 68551-07-5 1-Tridecanol 112-70-9 Alcohols, C14-16 68333-80-2 1-Tetradecanol 112-72-1 Alcohols, C6-12 68603-15-6 1-Pentadecanol 629-76-5 Alcohols, C12-16 68855-56-1 1-Hexadecanol 36653-82-4 Alcohols, C12-13 75782-86-4 CAS Nos 1-Eicosanol 629-96-9 Alcohols, C14-15 75782-87-5 1-Docosanol 661-19-8 Alcohols, C12-14 80206-82-2 Alcohols, C12-15 63393-82-8 Alcohols, C8-10 85566-12-7 Alcohols, C9-11 66455-17-2 Alcohols, C10-12 85665-26-5 Alcohols, C12-18 67762-25-8 Alcohols, C18-22 97552-91-5 9-Octadecen-1-ol 143-28-2 Alcohols, C14-18. 68155-00-0 (9Z)- & C16-18-unsatd Alcohols, C16-18 68002-94-8 Tridecanol, 90583-91-8 & C18 Unsaturated branched & linear Structural Formula CH3(CH2)nCH2OH Linear n = 4 to 20 CH3(CH2)nCHCH2OH 2-Alkyl branched n + m = 3 to 18, and m is predominantly (CH2)mCH3 = 0. Present in essentially-linear alcohols CH3(CH2)nCH(CH2)mOH Other-methyl branching n + m= 9 or 10 CH3 Present in essentially-linear Fischer- Tropsch derived alcohols CH3(CH2)7CH=CH(CH2)7CH2O Unsaturated H 9-Z unsaturated components are present in some commercial products. -

The Total Synthesis of Hexavalent Glycodendrimers
THE TOTAL SYNTHESIS OF HEXAVALENT GLYCODENDRIMERS USING A DIVERGENT PATHWAY FOR ANTI-HIV THERAPY A Thesis Presented to the faCulty of the Department of Chemistry California State University, SaCramento Submitted in partial satisfaCtion of the requirements for the degree of MASTER OF SCIENCE in Chemistry by Dustin Andres Dimas SUMMER 2018 © 2018 Dustin Andres Dimas ALL RIGHTS RESERVED ii THE TOTAL SYNTHESIS OF HEXAVALENT GLYCODENDRIMERS USING A DIVERGENT PATHWAY FOR ANTI-HIV THERAPY A Thesis by Dustin Andres Dimas Approved by: __________________________________, Committee Chair Dr. Katherine MCReynolds __________________________________, SeCond Reader Dr. Roy Dixon __________________________________, Third Reader Dr. Cynthia Kellen-Yuen ____________________________ Date iii Student: Dustin Andres Dimas I certify that this student has met the requirements for format contained in the University format manual, and that this thesis is suitable for shelving in the Library and credit is to be awarded for the thesis. __________________________, Graduate Coordinator ___________________ Dr. Susan Crawford Date Department of Chemistry iv AbstraCt of THE TOTAL SYNTHESIS OF HEXAVALENT GLYCODENDRIMERS USING A DIVERGENT PATHWAY FOR ANTI-HIV THERAPY by Dustin Andres Dimas The Human ImmunodefiCiency Virus (HIV) has caused a worldwide epidemiC. Currently, an estimated 36.9 million people are infeCted with HIV. The current treatment for HIV is antiretroviral therapy (ART). Around 20.9 million people living with HIV have aCCess to ART therapy. Right now, only 57% of the people infeCted are currently using ART. As of now, ART can only slow the progression of the virus but doesn’t cure the disease. Additionally, ART can have toxiC side effeCts or beCome ineffeCtive over time through viral resistance. -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

Aldehydes and Ketones
Organic Lecture Series Aldehydes And Ketones Chap 16 111 Organic Lecture Series IUPAC names • the parent alkane is the longest chain that contains the carbonyl group • for ketones, change the suffix -e to -one • number the chain to give C=O the smaller number • the IUPAC retains the common names acetone, acetophenone, and benzophenone O O O O Propanone Acetophenone Benzophenone 1-Phenyl-1-pentanone (Acetone) Commit to memory 222 Organic Lecture Series Common Names – for an aldehyde , the common name is derived from the common name of the corresponding carboxylic acid O O O O HCH HCOH CH3 CH CH3 COH Formaldehyde Formic acid Acetaldehyde Acetic acid – for a ketone , name the two alkyl or aryl groups bonded to the carbonyl carbon and add the word ketone O O O Ethyl isopropyl ketone Diethyl ketone Dicyclohexyl ketone 333 Organic Lecture Series Drawing Mechanisms • Use double-barbed arrows to indicate the flow of pairs of e - • Draw the arrow from higher e - density to lower e - density i.e. from the nucleophile to the electrophile • Removing e - density from an atom will create a formal + charge • Adding e - density to an atom will create a formal - charge • Proton transfer is fast (kinetics) and usually reversible 444 Organic Lecture Series Reaction Themes One of the most common reaction themes of a carbonyl group is addition of a nucleophile to form a tetrahedral carbonyl addition compound (intermediate). - R O Nu - + C O Nu C R R R Tetrahedral carbonyl addition compound 555 Reaction Themes Organic Lecture Series A second common theme is -

N-BUTYL ALCOHOL
Right to Know Hazardous Substance Fact Sheet Common Name: n-BUTYL ALCOHOL Synonyms: Propyl Carbinol; n-Butanol CAS Number: 71-36-3 Chemical Name: 1-Butanol RTK Substance Number: 1330 Date: November 1998 Revision: January 2008 DOT Number: UN 1120 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE n-Butyl Alcohol is a colorless liquid with a strong, sweet Hazard Summary alcohol odor. It is used as a solvent for fats, waxes, shellacs, Hazard Rating NJDOH NFPA resins, gums, and varnish, in making hydraulic fluids, and in HEALTH - 2 medications for animals. FLAMMABILITY - 3 REACTIVITY - 0 f ODOR THRESHOLD = 1 to 15 ppm FLAMMABLE f Odor thresholds vary greatly. Do not rely on odor alone to POISONOUS GASES ARE PRODUCED IN FIRE determine potentially hazardous exposures. CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; Reasons for Citation 4=severe f n-Butyl Alcohol is on the Right to Know Hazardous f n-Butyl Alcohol can affect you when inhaled and by Substance List because it is cited by OSHA, ACGIH, DOT, passing through the skin. NIOSH, DEP, IRIS, NFPA and EPA. f Contact can irritate and burn the skin. f This chemical is on the Special Health Hazard Substance f n-Butyl Alcohol can irritate and burn the eyes with possible List. eye damage. f Inhaling n-Butyl Alcohol can irritate the nose, throat and lungs. f Exposure to n-Butyl Alcohol can cause headache, dizziness, nausea and vomiting. SEE GLOSSARY ON PAGE 5. f n-Butyl Alcohol can damage the liver, kidneys, hearing, and sense of balance. -

Reactions of Alcohols & Ethers 1
REACTIONS OF ALCOHOLS & ETHERS 1. Combustion (Extreme Oxidation) alcohol + oxygen carbon dioxide + water 2 CH3CH2OH + 6 O2 4 CO2 + 6 H2O 2. Elimination (Dehydration) ° alcohol H 2 S O 4/ 1 0 0 C alkene + water H2SO4/100 ° C CH3CH2CH2OH CH3CH=CH2 + H2O 3. Condensation ° excess alcohol H 2 S O 4 / 1 4 0 C ether + water H2SO4/140 ° C 2 CH3CH2OH CH3CH2OCH2CH3 + H2O 4. Substitution Lucas Reagent alcohol + hydrogen halide Z n C l2 alkyl halide + water ZnCl2 CH3CH2OH + HCl CH3CH2Cl + H2O • This reaction with the Lucas Reagent (ZnCl2) is a qualitative test for the different types of alcohols because the rate of the reaction differs greatly for a primary, secondary and tertiary alcohol. • The difference in rates is due to the solubility of the resulting alkyl halides • Tertiary Alcohol→ turns cloudy immediately (the alkyl halide is not soluble in water and precipitates out) • Secondary Alcohol → turns cloudy after 5 minutes • Primary Alcohol → takes much longer than 5 minutes to turn cloudy 5. Oxidation • Uses an oxidizing agent such as potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7). • This reaction can also be used as a qualitative test for the different types of alcohols because there is a distinct colour change. dichromate → chromium 3+ (orange) → (green) permanganate → manganese (IV) oxide (purple) → (brown) Tertiary Alcohol not oxidized under normal conditions CH3 KMnO4 H3C C OH NO REACTION K2Cr2O7 CH3 tertbutyl alcohol Secondary Alcohol ketone + hydrogen ions H O KMnO4 H3C C CH3 + K2Cr2O7 C + 2 H H3C CH3 OH propanone 2-propanol Primary Alcohol aldehyde + water carboxylic acid + hydrogen ions O KMnO4 O H H KMnO H H 4 + CH3CH2CH2OH C C C H C C C H + 2 H K2Cr2O7 + H2O K Cr O H H H 2 2 7 HO H H 1-propanol propanal propanoic acid 6. -

Alcoholsalcohols
AlcoholsAlcohols Chapter 10 1 Structure of Alcohols • The functional group of an alcohol is H an -OH group bonded to an sp3 O 108.9° hybridized carbon. C H – Bond angles about the hydroxyl oxygen H H atom are approximately 109.5°. • Oxygen is sp3 hybridized. –Two sp3 hybrid orbitals form sigma bonds to a carbon and a hydrogen. – The remaining two sp3 hybrid orbitals each contain an unshared pair of electrons. 2 Nomenclature of Alcohols • IUPAC names – The parent chain is the longest chain that contains the OH group. – Number the parent chain to give the OH group the lowest possible number. – Change the suffix -etoe -ol.ol • Common names – Name the alkyl group bonded to oxygen followed by the word alcohol.alcohol 3 Nomenclature of Alcohols OH o o 1o OH 1 2 OH 1-Propanol 2-Propan ol 1-Bu tanol (Pro py l alco ho l) (Isoprop yl alcoh ol) (Bu tyl alcoh ol) OH 2o OH 1o OH 3o 2-Butanol 2-M eth yl-1-p ropan ol 2-M eth yl-2-p ropan ol (sec-Butyl alcohol) (Isobutyl alcohol) (tert -Butyl alcohol) 4 Nomenclature of Alcohols • Compounds containing more than one OH group are named diols, triols, etc. CH2 CH2 CH3 CHCH2 CH2 CHCH2 OH OH HO OH HO HO OH 1,2-Ethanediol 1,2-Propanediol 1,2,3-Propanetriol (Ethylene glycol) (Propylene glycol) (Glycerol, Glycerine) 5 Physical Properties • Alcohols are polar compounds. – They interact with themselves and with other polar compounds by dipole-dipole interactions. • Dipole-dipole interaction: The attraction between the positive end of one dipole and the negative end of another. -

Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers
Chapter 18 Ethers and Epoxides; Thiols and Sulfides Ethers • Ethers (R–O–R’): – Organic derivatives of water, having two organic groups bonded to the same oxygen atom © 2016 Cengage Learning 2 NAMES AND PROPERTIES OF ETHERS 3 Nomenclature: Common Names • Simple ethers are named by identifying two organic substituents and adding the word ether – Name the groups in alphabetical order – Symmetrical: Use dialkyl or just alkyl © 2016 Cengage Learning 4 Nomenclature: IUPAC Names • The more complex alkyl group is the parent name • The group with the oxygen becomes an alkoxy group © 2016 Cengage Learning 5 Nomenclature: Cyclic Ethers (Heterocycles) • Heterocyclic: Oxygen is part of the ring. O • Epoxides (oxiranes) H2C CH2 O • Oxetanes • Furans (Oxolanes) O O • Pyrans (Oxanes) O O O • Dioxanes O © 2013 Pearson Education, Inc. 6 Epoxide Nomenclature • Name the starting alkene and add “oxide” © 2013 Pearson Education, Inc. 7 Epoxide Nomenclature • The oxygen can be treated as a substituent (epoxy) on the compound • Use numbers to specify position • Oxygen is 1, the carbons are 2 and 3 • Substituents are named in alphabetical order © 2013 Pearson Education, Inc. 8 Properties of Ethers • Possess nearly the same geometry as water – Oxygen atom is sp3-hybridized – Bond angles of R–O–R bonds are approximately tetrahedral • Polar C—O bonds © 2013 Pearson Education, Inc. 9 Properties of Ethers: Hydrogen Bond • Hydrogen bond is a attractive interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom • Ethers cannot hydrogen bond with other ether molecules, so they have a lower boiling point than alcohols • Ether molecules can hydrogen bond with water and alcohol molecules • They are hydrogen bond acceptors © 2013 Pearson Education, Inc. -

Soln-2212-PT3-S20-Ch-16-18.Pdf
1. 2. H2SO4 H2SO4 H2SO41) BH3, THF 1) BH3, THF 1) BH3, THF - - - 2) OH , H2O2, H2O2) OH , H2O2, H2O 2) OH , H2O2, H2O OH OH OH 3. OH OH OH H2SO4 1) BH3, THF - 2) OH , H2O2, H2O OH PBr3 PBr3 PBr3 Pyridine Pyridine Pyridine OH PBr3 Pyridine O O O Mg Mg Mg HO HO HO1) 1) 1) Ether Ether Ether 2) HCl 2) HCl 2) HCl Br Br Br MgBr MgBr MgBr O Mg HO 1) Ether 2) HCl Br 4. MgBr **This reaction reduces an aldehyde to a primary alcohol and a ketone to a secondary alcohol. Since only one EQ of reagent is given the most reactive functional group will be reduced (in this case the aldehyde) **ketones are less reactive than aldehydes and the more steric hindrance surrounding the ketone the less reactive it is. Note: NaBH4 reduces ketones but is too weak to reduce an ester/double/triple bonds 5. ** With these reagents a tertiary amide will be reduced to a tertiary amine, a secondary amide will be reduced to a secondary amine, and a primary amide will be reduced to a primary amine 6. ** Recall from chapter 7, OChem 1 H2 and Lindlars catalyst always reduces an alkyne to a CIS ALKENE ** Li/Na and liquid NH3 always reduces an alkyne to a TRANS ALKENE 7. **A ketone/aldehyde reacts with primary amines in trace acid to produce an imine, and reacts with a secondary amine in trace acid to produce an enamine (double bond forms between the alpha and beta carbons) **Sodium cyanoborohydride (NaBH3CN) is used to reduce imines/enamines back to their respective secondary/tertiary amines 8. -

Group in Amine Protection and Activation Patrice Ribière, Valérie Declerck, Jean Martinez, Frédéric Lamaty
2-(Trimethylsilyl)ethanesulfonyl (or SES) Group in Amine Protection and activation Patrice Ribière, Valérie Declerck, Jean Martinez, Frédéric Lamaty To cite this version: Patrice Ribière, Valérie Declerck, Jean Martinez, Frédéric Lamaty. 2-(Trimethylsilyl)ethanesulfonyl (or SES) Group in Amine Protection and activation. Chemical Reviews, American Chemical Society, 2006, 106, pp.2249-2269. hal-00116881 HAL Id: hal-00116881 https://hal.archives-ouvertes.fr/hal-00116881 Submitted on 18 Dec 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Chem. Rev. 2006, 106, 2249−2269 2249 2-(Trimethylsilyl)ethanesulfonyl (or SES) Group in Amine Protection and Activation Patrice Ribie`re, Vale´rie Declerck, Jean Martinez, and Fre´de´ric Lamaty* Laboratoire des Aminoacides, Peptides et Prote´ines (LAPP), CNRS-Universite´s Montpellier 1 et 2, Place Euge`ne Bataillon, 34095 Montpellier Cedex 5, France Received July 25, 2005 Contents 7. SES Protection on Polymeric Support 2266 8. Conclusion 2268 1. Introduction 2249 9. Abbreviations 2268 2. SES−Cl in Synthesis 2250 10. References 2268 2.1. Synthesis of Sulfonyl Chloride 2250 2.2. Protection of Amines 2250 3. Direct Introduction of 2251 2-(Trimethylsilyl)ethanesulfonamide in Synthesis 1.