N-BUTYL ALCOHOL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Storage Cabinets
Storage cabinets Incompatible chemicals must be kept separately to reduce the risk of mixing in case of accidental breakage or response to an emergency within the laboratory. (A starting point is to check the Safety Data Sheet for hazard and storage requirements.) Containers must be kept tightly closed and stored in cabinets which are suitable for the chemical. It is important to check materials which have a shelf life and safely dispose of before the date expires, and generally check the label to ensure it is still suitable and provides the necessary safety information. The condition of the cabinets needs to be checked to ensure that they are providing adequate containment of the materials being stored. Ensure spill trays are used which are capable of holding 110% volume of the largest container being stored in the tray. There may be occasions where the quantities being stored are very small and secondary containment of the chemicals within a cabinet provided would be deemed as providing adequate separation. The table below provides guidance on the type of materials being stored and the type of cabinet which is suitable for that material. This is not a definitive list and does not include ‘Highly Toxic’ materials. Separate guidance is in development. Hazard Group Type of cabinet Safety Sign and suggested Other information Max allowed (where wording applicable) Flammable Liquids Metal flammable storage Flammable liquids must 50 litres of highly flammable cabinet to BS EN 14470- never be stored in a liquid stored in any one E.g. alcohols, toluene, 1:2004, offering minimum refrigerator or freezer laboratory. -

Treating Irrigation Systems with Chlorine 1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. CIR1039 Treating Irrigation Systems with Chlorine 1 Kati W. Migliaccio, Brian Boman and Gary A. Clark2 Introduction Irrigation systems can become partially or completely clogged from biological growths of bacteria or algae which are often present in surface water and ground water. Bacteria and algae use chemical elements such as nitrogen, phosphorus, sulfur, or iron as nutrient sources to grow and develop (Figures 1 and 2) (Pitts et al., 2003). Thus, irrigation systems that also receive nutrients (such as natural background concentrations in the water or from fertigation) may experience greater rates of clogging. While all irrigation systems should have some Figure 1. Irrigation emitter clogged with algal growth. type of filtration system, this alone cannot effectively Credits: Brian Boman UF/IFAS remove microorganisms. Microorganism growth can result in clogged pipes, fittings, and emission devices of the chlorine chemical must be used to provide an (sprinklers, drippers, spray jets, etc.), decreasing effective water treatment program without damaging water application amounts and reducing application the irrigation system or the agricultural crop. uniformity and efficiency. The results of these are This publication provides a guide for using generally reduced agriculture productivity. A chlorine to treat inhibiting microorganism buildup in chemical method for removing microbial growth is irrigation systems. chlorination. Proper injection methods and amounts 1. This document is CIR1039, one of a series of the Agricultural and Biological Engineering Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Original publication date July 1992. -

186 SESSION LAWS [Chap
186 SESSION LAWS [Chap. Sec. 2. This act shall take effect and be in force from and after its passage. Approved April 13, 1925. CHAPTER 187—S. F. No. 984. An act to safeguard the distribution and sale of certain danger- ous caustic or corrosive acids, alkalis, and other substances. Be it enacted by the Legislature of the State of Minnesota: Section 1. Definitions.—That in this act, unless the context or subject-matter otherwise requires, A. The term "dangerous caustic or corrosive substance" means each and all of the acids, alkalis, and substances named below: (a) Hydrochloric acid and any preparation containing free or chemi- cally unneutralized hydrochloric acid (HC1) in a concentration of ten per centum or more; (b) Sulphuric acid and any preparation containing free or chemically unneulralized sulphuric acid (H2SO4) in a concentration of ten per centum or more; (c) Nitric acid or any preparation containing free or chemically unneutralized nitric acid (HNO3) in a concentration of five per centum or more; (d) Carbolic acid (C6H5OH), otherwise known as phenol, and any preparation containing carbolic acid in a concentration of five per centum or more; (e) Oxalic acid and any preparation containing free or chemically unneutralized oxalic acid (H2C2O4) in a con- centration of ten per centum or more; (f) Any salt of oxalic acid and any preparation containing any such salt in a concentration of ten per centum or more; (g) Acetic acid or any preparation con- taining free or chemically unneutralized acetic acid (HC2H3O2) in a concentration of -

SAFETY DATA SHEET Isopropyl Alcohol
SAFETY DATA SHEET Isopropyl Alcohol Section 1. Identification GHS product identifier : Isopropyl Alcohol Chemical name : Isopropyl alcohol Other means of : isopropanol; 2-Propanol identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. Synonym : isopropanol; 2-Propanol SDS # : 001105 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture EYE IRRITATION - Category 2A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Highly flammable liquid and vapor. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Use only outdoors or in a well-ventilated area. Avoid breathing vapor. Wash hands thoroughly after handling. Response : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or physician if you feel unwell. -

Enhanced Butanol Production by Free and Immobilized Clostridium Sp
Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells Using Butyric Acid as Co-Substrate Laili Gholizadeh This thesis comprises 30 ECTS credits and is a compulsory part in the Master of Science with a Major in Chemical Engineering – Applied Biotechnology 120 ECTS credits No. 10/2009 Title: Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells using Butyric Acid as Co-Substrate. Author: Laili Gholizadeh Baroghi (e-mail: [email protected]) Master Thesis Subject Category: Biotechnology (Bioprocess Engineering – Biofuels) University College of Borås School of Engineering SE-501 90 BORÅS Telephone: (+46) 033 435 4640 Examiner: Prof. Mohammad Taherzadeh Supervisor and Thesis Advisor: Prof. Shang–Tian Yang Supervisor Address: OSU–Ohio State University 125 Koffolt Laboratories 140 West 19th Ave. Columbus, OH 43210–1185, USA Client: Ohio State University (OSU), Chemical & Biomolecular Engineering Department Prof. Shang–Tian Yang Columbus, Ohio; USA. Date: 08–12–2009 Keywords: Bio-butanol y Acetone–Butanol–Ethanol (ABE) y ABE- fermentation y Butyric acid y Clostridium y C. acetobutylicum ATCC 824 y C. beijerinckii ATCC 55025 y C. beijerinckii BA 101 y C. beijerinckii NCIMB 8052 y Fibrous-bed Bioreactor (FBB) y Batch y Suspended cell culture y Immobilized cell system. DEDICATION I would like to dedicate this M.Sc. Thesis to my beloved Family for all their love and encouragement and for always been supportive of my choices. “I am among those who think that science has great beauty. A scientist in his laboratory is not only a technician: he is also a child placed before natural phenomena, which impress him like a fairy tale.” − Marie Curie ABSTRACT Butanol production by four different Clostridium sp. -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

Aldehydes and Ketones
Organic Lecture Series Aldehydes And Ketones Chap 16 111 Organic Lecture Series IUPAC names • the parent alkane is the longest chain that contains the carbonyl group • for ketones, change the suffix -e to -one • number the chain to give C=O the smaller number • the IUPAC retains the common names acetone, acetophenone, and benzophenone O O O O Propanone Acetophenone Benzophenone 1-Phenyl-1-pentanone (Acetone) Commit to memory 222 Organic Lecture Series Common Names – for an aldehyde , the common name is derived from the common name of the corresponding carboxylic acid O O O O HCH HCOH CH3 CH CH3 COH Formaldehyde Formic acid Acetaldehyde Acetic acid – for a ketone , name the two alkyl or aryl groups bonded to the carbonyl carbon and add the word ketone O O O Ethyl isopropyl ketone Diethyl ketone Dicyclohexyl ketone 333 Organic Lecture Series Drawing Mechanisms • Use double-barbed arrows to indicate the flow of pairs of e - • Draw the arrow from higher e - density to lower e - density i.e. from the nucleophile to the electrophile • Removing e - density from an atom will create a formal + charge • Adding e - density to an atom will create a formal - charge • Proton transfer is fast (kinetics) and usually reversible 444 Organic Lecture Series Reaction Themes One of the most common reaction themes of a carbonyl group is addition of a nucleophile to form a tetrahedral carbonyl addition compound (intermediate). - R O Nu - + C O Nu C R R R Tetrahedral carbonyl addition compound 555 Reaction Themes Organic Lecture Series A second common theme is -

N-Hexyl-Chloroformate-NHEXCF.Pdf
Date of issue: 11. 02. 2004. Date of revision: 15.01.2015. Version: CLP_ C msds-europe.com SAFETY DATA SHEET SECTION 1 : IDENTIFICATION OF THE SUBSTANCE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier: HEXYL CHLOROFORMATE Synonym: Chloroformic Acid Hexyl Ester CAS number: 6092-54-2 EU number: 228-036-4 Index number: 607-064-00-4 Registration number: Registration number is not available for this substance since this substance or its use is exempted from registration based on Article 2 of REACH regulation, or registration is not necessary due to the annual tonnage band, or the registration is scheduled to a later registration date. 1.2. Relevant identified uses of the substance and uses advised against: Organic synthesis intermediate for industrial use. 1.3. Details of the supplier of the safety data sheet: FRAMOCHEM FRENCH-HUNGARIAN FINE CHEMICALS LTD. 3700 Kazincbarcika, Szerviz str. 5., POB. 504 Telephone: +36 (48) 311-991 Fax: +36 (48) 512-162 1.3.1. Responsible person: - E-mail: [email protected] 1.4. Emergency telephone number: Public Toxicological Health Service (ETTSZ) 1096 Budapest, Nagyvárad tér 2. Tel.: 06 1 476 6464, 06 80 201 199 (0-24 h) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance: Classification according to Regulation 1272/2008/EC (CLP): Acute Tox (oral) 3 – H301 Acute Tox. 3 (dermal) – H311 Skin Corr. 1B – H314 Acute Tox. (inhalative) – H331 Warning H statements: H301 – Toxic if swallowed. H311 – Toxic in contact with skin. H314 – Causes severe skin burns and eye damage. H331 – Toxic if inhaled. Classification according to Directive 67/548/EEC: T; Toxic – R 23/24/25 C; Corrosive – R34 R phrases referring to the hazards/risks: FRAMOCHEM FRENCH-HUNGARIAN FINE CHEMICALS LTD. -
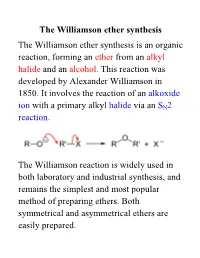
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

How Is Alcohol Metabolized by the Body?
Overview: How Is Alcohol Metabolized by the Body? Samir Zakhari, Ph.D. Alcohol is eliminated from the body by various metabolic mechanisms. The primary enzymes involved are aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), cytochrome P450 (CYP2E1), and catalase. Variations in the genes for these enzymes have been found to influence alcohol consumption, alcohol-related tissue damage, and alcohol dependence. The consequences of alcohol metabolism include oxygen deficits (i.e., hypoxia) in the liver; interaction between alcohol metabolism byproducts and other cell components, resulting in the formation of harmful compounds (i.e., adducts); formation of highly reactive oxygen-containing molecules (i.e., reactive oxygen species [ROS]) that can damage other cell components; changes in the ratio of NADH to NAD+ (i.e., the cell’s redox state); tissue damage; fetal damage; impairment of other metabolic processes; cancer; and medication interactions. Several issues related to alcohol metabolism require further research. KEY WORDS: Ethanol-to acetaldehyde metabolism; alcohol dehydrogenase (ADH); aldehyde dehydrogenase (ALDH); acetaldehyde; acetate; cytochrome P450 2E1 (CYP2E1); catalase; reactive oxygen species (ROS); blood alcohol concentration (BAC); liver; stomach; brain; fetal alcohol effects; genetics and heredity; ethnic group; hypoxia The alcohol elimination rate varies state of liver cells. Chronic alcohol con- he effects of alcohol (i.e., ethanol) widely (i.e., three-fold) among individ- sumption and alcohol metabolism are on various tissues depend on its uals and is influenced by factors such as strongly linked to several pathological concentration in the blood T chronic alcohol consumption, diet, age, consequences and tissue damage. (blood alcohol concentration [BAC]) smoking, and time of day (Bennion and Understanding the balance of alcohol’s over time. -

Alcoholsalcohols
AlcoholsAlcohols Chapter 10 1 Structure of Alcohols • The functional group of an alcohol is H an -OH group bonded to an sp3 O 108.9° hybridized carbon. C H – Bond angles about the hydroxyl oxygen H H atom are approximately 109.5°. • Oxygen is sp3 hybridized. –Two sp3 hybrid orbitals form sigma bonds to a carbon and a hydrogen. – The remaining two sp3 hybrid orbitals each contain an unshared pair of electrons. 2 Nomenclature of Alcohols • IUPAC names – The parent chain is the longest chain that contains the OH group. – Number the parent chain to give the OH group the lowest possible number. – Change the suffix -etoe -ol.ol • Common names – Name the alkyl group bonded to oxygen followed by the word alcohol.alcohol 3 Nomenclature of Alcohols OH o o 1o OH 1 2 OH 1-Propanol 2-Propan ol 1-Bu tanol (Pro py l alco ho l) (Isoprop yl alcoh ol) (Bu tyl alcoh ol) OH 2o OH 1o OH 3o 2-Butanol 2-M eth yl-1-p ropan ol 2-M eth yl-2-p ropan ol (sec-Butyl alcohol) (Isobutyl alcohol) (tert -Butyl alcohol) 4 Nomenclature of Alcohols • Compounds containing more than one OH group are named diols, triols, etc. CH2 CH2 CH3 CHCH2 CH2 CHCH2 OH OH HO OH HO HO OH 1,2-Ethanediol 1,2-Propanediol 1,2,3-Propanetriol (Ethylene glycol) (Propylene glycol) (Glycerol, Glycerine) 5 Physical Properties • Alcohols are polar compounds. – They interact with themselves and with other polar compounds by dipole-dipole interactions. • Dipole-dipole interaction: The attraction between the positive end of one dipole and the negative end of another. -

July 2014 1/15 ASSESSMENT of the PERFORMANCE
July 2014 ASSESSMENT OF THE PERFORMANCE CHARACTERISTICS OF PROPOSED IN VITRO MEMBRANE BARRIER TEST METHOD FOR SKIN CORROSION This document contains Performance Standards (PS) for determining the validation status (reliability and relevance) of similar and modified skin corrosion test methods that are structurally and mechanistically similar to the in vitro membrane barrier test method in OECD Test Guideline 435 (1), in accordance with the principles of Guidance Document No. 34 (3). These PS include a list of 40 Reference Chemicals by which to evaluate assay performance, the essential test method components by which to evaluate the structural, mechanistic and procedural similarity of a new proposed test method, and the minimum reliability and accuracy values necessary for the test method to be considered comparable to the validated reference method (VRM). INTRODUCTION 1. Test methods proposed for use under this Test Guideline should be evaluated to determine their Field Code Changed reliability and accuracy using substances of known corrosivity and non-corrosivity. When evaluated using the recommended reference chemicals (Table 1), the proposed test methods should have reliability and accuracy that are comparable to that of the validated reference test method (VRM) (2)(Table 2). The reliability and accuracy standards that should be achieved are provided in paragraphs 3 and 4. Non- corrosive and corrosive substances, ranging from strong to weak and representing relevant chemical classes are included so that the reliability and accuracy, i.e., sensitivity, specificity, false negative rates, and false positive rates, of the proposed test method can be compared to that of the validated reference test method (2)(3).