Photosynthesis Photosynthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comedy of Scientific Errors BOVINES
SACRED A COMEDY OF SCIENTIFIC ERRORS BOVINES DOUGLAS ALLCHIN, DEPARTMENT EDITOR William Shakespeare may well have foreshadowed the modern television experiment with balm, groundsel, and spinach. All modified the air to sitcom. His comic misadventures were expertly crafted. In A Comedy of support sustained burning. Animals, too, could breathe longer in the Errors, for example, twins (with twin servants), each separated at birth, treated air. Plants, Priestley had found, could restore the “goodness” of converge unbeknownst to each other in the same town. Mistaken iden- the air depleted by respiration or combustion. American correspondent tity leads to miscommunication. More mistaken identity follows, with Benjamin Franklin immediately perceived the global implications: plants more misdelivered messages and yet more misinterpretations. Hilarious help restore the atmosphere that humans and other animals foul. The consequences ensue. It is a stock comedic formula in modern entertain- system ensures our survival. That view fit neatly with Priestley’s religious ment. A character first makes an unintentional error. Then ironically, in belief in an intentionally designed (and rational) natural world. It was a trying to correct it, things only get laughably worse. remarkable discovery. For this and other work on airs, the Royal Society Science, we imagine, is safeguarded against such embarrassing in 1772 awarded Priestley the Copley Medal, then the most prestigious episodes. In the lore of scientists, echoed among teachers, science is honor in science. “self-correcting.” Replication, in particular, ensures that errors are Others were eager to build on Priestley’s discovery about plants and exposed for what they are. Research promptly returns to its fruitful tra- the restoration of air. -

Which Regions of the Electromagnetic Spectrum Do Plants Use to Drive Photosynthesis?
Which regions of the electromagnetic spectrum do plants use to drive photosynthesis? Green Light: The Forgotten Region of the Spectrum. In the past, plant physiologists used green light as a safe light during experiments that required darkness. It was assumed that plants reflected most of the green light and that it did not induce photosynthesis. Yes, plants do reflect green light but human vision sensitivity peaks in the green region at about 560 nm, which allows us to preferentially see green. Plants do not reflect all of the green light that falls on them but they reflect enough for us to detect it. Read on to find out what the role of green light is in photosynthesis. The electromagnetic spectrum: Light Visible light ranges from low blue to far-red light and is described as the wavelengths between 380 nm and 750 nm, although this varies between individuals. The region between 400 nm and 700 nm is what plants use to drive photosynthesis and is typically referred to as Photosynthetically Active Radiation (PAR). There is an inverse relationship between wavelength and quantum energy, the higher the wavelength the lower quantum energy and vice versa. Plants use wavelengths outside of PAR for the phenomenon known as photomorphogenesis, which is light regulated changes in development, morphology, biochemistry and cell structure and function. The effects of different wavelengths on plant function and form are complex and are proving to be an interesting area of study for many plant scientists. The use of specific and adjustable LEDs allows us to tease apart the roles of specific areas of the spectrum in photosynthesis. -

Development of Preservice Biology Teachers‟ Skills in the Causal Process Concerning Photosynthesis
Journal of Education and Training Studies Vol. 7, No. 4; April 2019 ISSN 2324-805X E-ISSN 2324-8068 Published by Redfame Publishing URL: http://jets.redfame.com Development of Preservice Biology Teachers‟ Skills in the Causal Process Concerning Photosynthesis Arzu Saka Correspondence: Arzu Saka, Trabzon University, Fatih Faculty of Education, Trabzon, 61335,Turkey. Received: February 12, 2019 Accepted: March 7, 2019 Online Published: March 11, 2019 doi:10.11114/jets.v7i4.4022 URL: https://doi.org/10.11114/jets.v7i4.4022 Abstract Photosynthesis is the most effective cycle and sustainable natural process known in nature. Students who learn the subject of photosynthesis well will also make better sense of other issues such as environmental problems, the state of the atmosphere, greenhouse gases, climate changes, carbon footprints and conservation of forests. The aim of this study is to present an example of a worksheet that investigates the skill levels possessed by preservice teachers‟ in the causal process, and also to examine their ability to write a photosynthesis equation by means of a history-based approach that also includes reading skills. The study was conducted with the action research method. The study sample consisted of a total of 71 preservice biology teachers. At the first stage, before the implementation of the worksheet, 34 teacher candidates from within the sample were asked a question with a diagram summarising the photosynthesis process. At the second stage, all the prospective teachers were asked for the skill levels they possessed in the causal process to be assessed via implementation of the worksheet. The answers given by the preservice teachers in the defining variables section in particular make up the least answered section of the worksheet. -
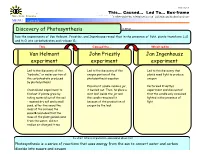
Discovery of Photosynthesis Jan Ingenhousz Experiment John
Cause / Effect TM This… Caused… Led To… Box-frame . Makes Sense Strategies © 2007 Edwin Ellis, All Rights© 2007 Reserved Edwin Published Ellis, byAll Makes Rights Sense Reserved Strategies, LLC, www.MakesSenseStrategies.com Lillian, AL www.MakesSenseStrategies.com MENU sample Name: Date: Discovery of Photosynthesis Is about … how the experiments of Van Helmont, Priestley, and Ingenhousz reveal that in the presence of light, plants transform C2O and H2O into carbohydrates and release O2. This Caused the… Which led to … Van Helmont John Priestly Jan Ingenhousz experiment experiment experiment · Led to the discovery of the · Led to the discovery of the · Led to the discovery that “hydrate,” or water portion of oxygen portion of the plants need light to produce the carbohydrate produced photosynthesis equation oxygen by photosynthesis · Placed a lit candle inside a jar, · Performed Priestly’s · Created and experiment to it burned out. Then, he place a experiment and discovered find out if plants grew by mint leaf inside the jar and that the candle only remained taking material out of the soil the candle remained lit lighted in the presence of – massed dry soil and a small because of the production of light seed, after five years the oxygen by the leaf mass of the soil was the sameèconcluded that the mass of the plant gained came from the water, did not realize air changed it too So what? What is important to understand about this? Photosynthesis is a series of reactions that uses energy from the sun to convert water and carbon dioxide into sugars and oxygen . -

Photobiology
Photobiology Jode Himann |Nemalux Research | 12.01.2017 Photobiology is the study of light and its effect on living things. There has been a great deal of study on photobiology in the area of agriculture. This interest is growing because of the science becoming more refined and the marketplace developing. Some random notes and an initial Nemalux fixture spectra is shown. McCree Curve: K. J. McCree (1970) studied and developed the action spectrum, absorption and quantum yield curves of photosynthesis in crop plants. The action spectrum is commonly known as the McCree Curve. McCree curve shows the wavelengths shows the relative photosynthesis response in the between 400-700 nm (commonly known as photosynthetically active region or PAR) for plants growth. This is also known as generalized PAR curve. McCree also showed the relative quantum yield and relative absorption for 400-700nm region. This work has been the basis of all of the researches that followed until now. 1 Image : Econolux Action curve vs Absorption curve: Action curve shows the wavelengths that are most effective for photosynthesis. On the other hand Absorption curve shows the wavelengths that are absorbed by Chlorophylls (Chlorophyll A & B) and Cartenoids. Image : Econolux It is easily understood that most of the absorption happens in 400-500nm (blue) and 600-700nm (red) region. There’s a very little absorbance in the 500-600nm (green) region. However, there are still 70% of the 550nm absorptance is present as the integrity of leaf increases. Different studies shows that Chlorophylls responds to Blue (400 – 500nm) and Red (600-700nm) wavelengths. -

Jean Senebier's Thoughts on Experimentation
| Jean Senebier’s thoughts on experimentation and their relevance for today’s researcher | 185 | Jean Senebier’s thoughts on experimentation and their relevance for today’s researcher Edward E. FARMER * Ms. received the 22nd April 2010, accepted the 10th September 2010 T Abstract How are observation and experimentation related to one another? Jean Senebier (1742-1809) tackled this question in his philosophical works on The Art of Observing. However, Senebier was not only a theoretician and, not long after his first publications on observation, his own experiments contributed to resolve a major question in biology: what do the leaves of plants feed on? By analysing Senebier’s works on science theory in parallel with those reporting his scientific discoveries, this article shows that The Art of Observing series is not restricted to observation and contains deep insights into the process of experimenting with living organisms. Keywords: Senebier, plant physiology, photosynthesis, science didactics, epistemology T Résumé Les considérations de Jean Senebier sur l’expérimentation et leur intérêt pour les chercheurs d’aujourd’hui. – Quel est le lien entre l’observation et l’expérimentation? Jean Senebier (1742-1809) a abordé cette question dans sa série d’ouvrages sur l’Art d’observer. Cependant, Senebier n’était pas seulement un théoricien et, peu après ses premières publi - cations sur l’observation, il a commencé à travailler sur l’une des grandes questions de la biologie: « de quoi se nourrissent les feuilles des plantes? » S’appuyant sur ce qu’il avait appris en tant qu’expérimentaliste, Senebier a par la suite été en mesure d’approfondir ses brillantes analyses présentées dans ses deux premières publications sur l’Art d’observer. -

Measuring the Short-Term Plant Photosynthetic Response to Varying Light Quality Using Light Emitting Diodes (Leds)
Measuring the Short-term Plant Photosynthetic Response to Varying Light Quality Using Light Emitting Diodes (LEDs) Michael A. Schwalb Department of Bioresource Engineering McGill University Montreal, Quebec, Canada A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Master of Science December, 2013 © Michael Schwalb, 2013 1 Abstract Light emitting diodes (LEDs) emit narrow bandwidth light and have the potential to increase the spectral efficiency of supplemental lighting in greenhouses by optimizing spectral output for plant growth and yields. At the moment of writing, data describing the plant response to varying light quality and quantity was limited. The objective of this research was to examine photosynthetic response of plants to varying light quality and quantity and to gather photosynthetic response data that could be used to design an optimal spectrum for a prototype LED array for plant growth experiments. The action spectrum of tomato (Solanum lycopersicum), lettuce (Lactuca sativa) and petunia (Petunia × hybrida) seedlings was measured at three irradiances (30, 60 and 120 µmol m-2 sec-1) using LED arrays with peak wavelengths from 405nm – 700nm and a bandwidth of 25nm (full width at half maximum). The action spectrums for all plant species at all three irradiances were characterized by localized blue and red action peaks within the range of 430 to 449 nm and 624 to 660 nm respectively. A peak also occurred at 595 nm for 30 µmol m-2 sec-1.The photosynthetic response of tomato, lettuce and petunia to varying red (660nm) and blue (430nm) wavelengths with and without background broadband radiation was also measured. -

Printable PDF: RGB Light Source And
6400-18 RGB Light Source & 5 Photosynthetic Assimilation Summary non-photochemical processes (e.g. heat; ● The 6400-18 Red, Green, Blue (RGB) Blankenship, 2002). However, because other Light Source can deliver 2000 μmol m-2 wavelengths of light are absorbed and are s-1 white light†. equally capable of driving photosynthesis, it ● Three LED colors can be controlled is important to not only describe an absorp- independently to deliver any combina- tion spectrum, but also the photosynthetic tion desired. action spectrum for the absorbed light. OTE OTE ● Absorption spectrum of a plant drives the effectiveness of each color’s photosyn- Action spectra describe the amount of CO2- thetic response. fixed or O2-released at a particular wave- ● LED light should be normalized to the length across the absorption spectrum for a absorption spectrum of the plant. leaf. As is the case for absorption spectra, N N action spectra vary greatly between species Action spectrum of plants and RGB (McCree, 1972). For many C3 species, there is greater assimilation when plants are output illuminated with red light and to a lesser Not all incident radiation is absorbed by extent blue light than when illuminated with actively photosynthesizing leaves. Depending green light. on wavelength, light incident on a leaf may be reflected, transmitted through the leaf or The 6400-18 Red, Green, Blue (RGB) Light absorbed by the light harvesting complex to Source is a composite LED source comprised drive the light reactions. The proportion of of multiple diodes embedded within a tile. light absorbed at any particular wavelength The LED wavelength peaks of this commer- varies between and within species, and cially available tile are 460, 522 and 635 ± 5 frequently between individual leaves in a nm, corresponding to light in the blue, green plant canopy, dependent on canopy position and red regions of the light spectrum respec- and the history of the leaf. -

Horticulator Photosynthetic Active Radiation (PAR)
Application Note Vorteile Horticulturevon LED-Beleuchtung in | Microcosm GartenbauanwendungenLED it grow! Dies hat zur Folge, dass der Wirkungsgrad sehr stark von den Wellenlängen zu hohe oder zu geringe Mengen liefern (Abbildung 5). Strompreisen abhängig ist (Abbildung 4). Mit steigendem Strompreis Außerdem kann die Lichtrezeptur nicht an eine Pflanzenentwicklung werden die Einsparungen bei der Implementierung eines LED- angepasst werden (Abbildung 6). Derzeit gibt es eine Reihe von Projekten, Beleuchtungssystems deutlich größer. die die Lichtrezeptur (und andere Parameter) auf die Wachstumsphase der Pflanze abstimmen. Dabei kommen Kameras zum Einsatz, meist im 4 Lichtqualität sichtbaren oder infraroten Bereich. Der ultraviolette Bereich (UVA und UVB, 280 bis 400 nm) ist derzeit ein sehr interessantes Thema im Der Hauptvorteil von LEDs liegt in der Möglichkeit, das Gesamtspektrum Pflanzenanbau. Sonnenlicht besteht aus 9 % UV (Prozent PPF), während des Lichts anzupassen und zu optimieren. Dies kann genutzt werden, um HID-Quellen einen festen Wert von 0,3 bis 8 % UV-Strahlung (Prozent die photosynthetische Effizienz zu erhöhen und zu verbessern und PPF) emittieren[10]. Mit LEDs lässt sich die Belichtung sehr einfach steuern. Entwicklungsphasen zu steuern[8] aber auch, um die Menge an Eine unzureichende UV-Strahlung kann bei einigen Pflanzenarten die ungenutztem Licht und1. damit Essence Energie zu of reduzieren. Light Aufgrund ihrer Entwicklung3. What unterbrechen does [11]a. HIDplant-Quellen need? haben eine minimale fernrote monochromatischen Leistung können mehrere LEDs mit Strahlung (710 bis 740 nm), die LEDs effizient erzeugen können. unterschiedlichen WellenlängenLight is the verwendet visible part werden, of the um electromagnetic art-, sorten- und DieThe Be dimportanteutung d eparameterr fernrote nis S Photosynthetictrahlung wird i n ANO004 erklärt. -

The 100 Most Influential Scientists of All Time / Edited by Kara Rogers.—1St Ed
Published in 2010 by Britannica Educational Publishing (a trademark of Encyclopædia Britannica, Inc.) in association with Rosen Educational Services, LLC 29 East 21st Street, New York, NY 10010. Copyright © 2010 Encyclopædia Britannica, Inc. Britannica, Encyclopædia Britannica, and the Thistle logo are registered trademarks of Encyclopædia Britannica, Inc. All rights reserved. Rosen Educational Services materials copyright © 2010 Rosen Educational Services, LLC. All rights reserved. Distributed exclusively by Rosen Educational Services. For a listing of additional Britannica Educational Publishing titles, call toll free (800) 237-9932. First Edition Britannica Educational Publishing Michael I. Levy: Executive Editor Marilyn L. Barton: Senior Coordinator, Production Control Steven Bosco: Director, Editorial Technologies Lisa S. Braucher: Senior Producer and Data Editor Yvette Charboneau: Senior Copy Editor Kathy Nakamura: Manager, Media Acquisition Kara Rogers: Senior Editor, Biomedical Sciences Rosen Educational Services Jeanne Nagle: Senior Editor Nelson Sá: Art Director Introduction by Kristi Lew Library of Congress Cataloging-in-Publication Data The 100 most influential scientists of all time / edited by Kara Rogers.—1st ed. p. cm.—(The Britannica guide to the world’s most influential people) “In association with Britannica Educational Publishing, Rosen Educational Services.” Includes index. ISBN 978-1-61530-040-2 (eBook) 1. Science—Popular works. 2. Science—History—Popular works. 3. Scientists— Biography—Popular works. I. Rogers, Kara. -

Evolution of Photosynthesis
Evolution of photosynthesis The evolution of photosynthesis refers to the origin and subsequent evolution of photosynthesis, the process by which light energy synthesizes sugars from carbon dioxide and water, releasing oxygen as a waste product. The process of photosynthesis was discovered by Jan Ingenhousz, a Dutch-born British physician and scientist, first publishing about it in 1779.[1] The first photosynthetic organisms probably evolved early in the evolutionary history of life and most likely used reducing agents such as hydrogen or electrons, rather than water.[2] There are three major metabolic pathways by which photosynthesis is carried out: C3 photosynthesis, C4 photosynthesis, and CAM photosynthesis. C3 photosynthesis is the oldest and most common form. C3 is a plant that uses the calvin cycle for the initial steps that incorporate CO2 into organic material. C4 is a plant that prefaces the calvin cycle with reactions that incorporate CO2 into four-carbon compounds. CAM is a plant that uses crassulacean acid metabolism, an adaptation for photosynthesis in arid conditions. C4 and CAM Plants have special adaptations that save water.[3] Contents Origin Symbiosis and the origin of chloroplasts Evolution of photosynthetic pathways Concentrating carbon Evolutionary record When is C4 an advantage? See also References Origin The biochemical capacity to use water as the source for electrons in photosynthesis Life timeline evolved in a common ancestor of extant Ice Ages Quater n0ar y— Primates ← [4] Flowers Earliest apes cyanobacteria. The geological record P Birds h Mammals – Plants Dinosaurs indicates that this transforming event took Karo o a n ← Andean Tetrapoda place early in Earth's history, at least 2450– -50 0 — e Arthropods Molluscs r ←Cambrian explosion 2320 million years ago (Ma), and, it is o ← Cryoge nian Ediacara biota [5][6] – z ← speculated, much earlier. -

An Unfolding Discovery
Proc. Nat. Acad. Sci. USA Vol. 68, No. 11, pp. 2875, November 1971 Photosynthesis Bicentennial Symposium: Introduction by the Chairman KENNETH V. THIMANN Crown College, University of California, Santa CruG, Calif. 95060 In early August 1771 Joseph Priestley, chemist of Birmingham, mainly from the underside of the leaves. But the biological England, and codiscoverer of oxygen, performed his famous "climate" was not ready for the correct interpretation of the experiment with the mouse and the mint plant. This experi- bubbles and Bonnet's discussion was wholly in terms of heat- ment provided the beginnings of our understanding of that ing and the consequent expansion of gases at the leaf surface. remarkable process whereby the organic matter of our bio- In the following symposium Eugene Rabinowitch presents sphere is produced and our atmosphere continuously purified. It is perhaps not widely known that the discovery was al- the sequential development of the leading ideas in the action most made some 20 years earlier by Joseph Bonnet, another of light on leaves, and then the current developments in the very active researcher of the time. For Bonnet observed that physiological, biochemical and photochemical aspects of the when leaves were immersed in water and exposed to sunlight, field at the present day are summarized by some of the leaders bubbles of gas were produced. He even noted that they came in the study of these aspects of photosynthesis in this country. Proc. Nat. Acad. Sci. USA Vol. 68, No. 11, pp. 2875-2876, November 1971 An Unfolding Discovery EUGENE RABINOWITCH State University of New York, Albany, New York, 12203 About 1648, a Dutch alchemist, van Helmont, grew a willow "Experiments and Observations on Different Kinds of Air".