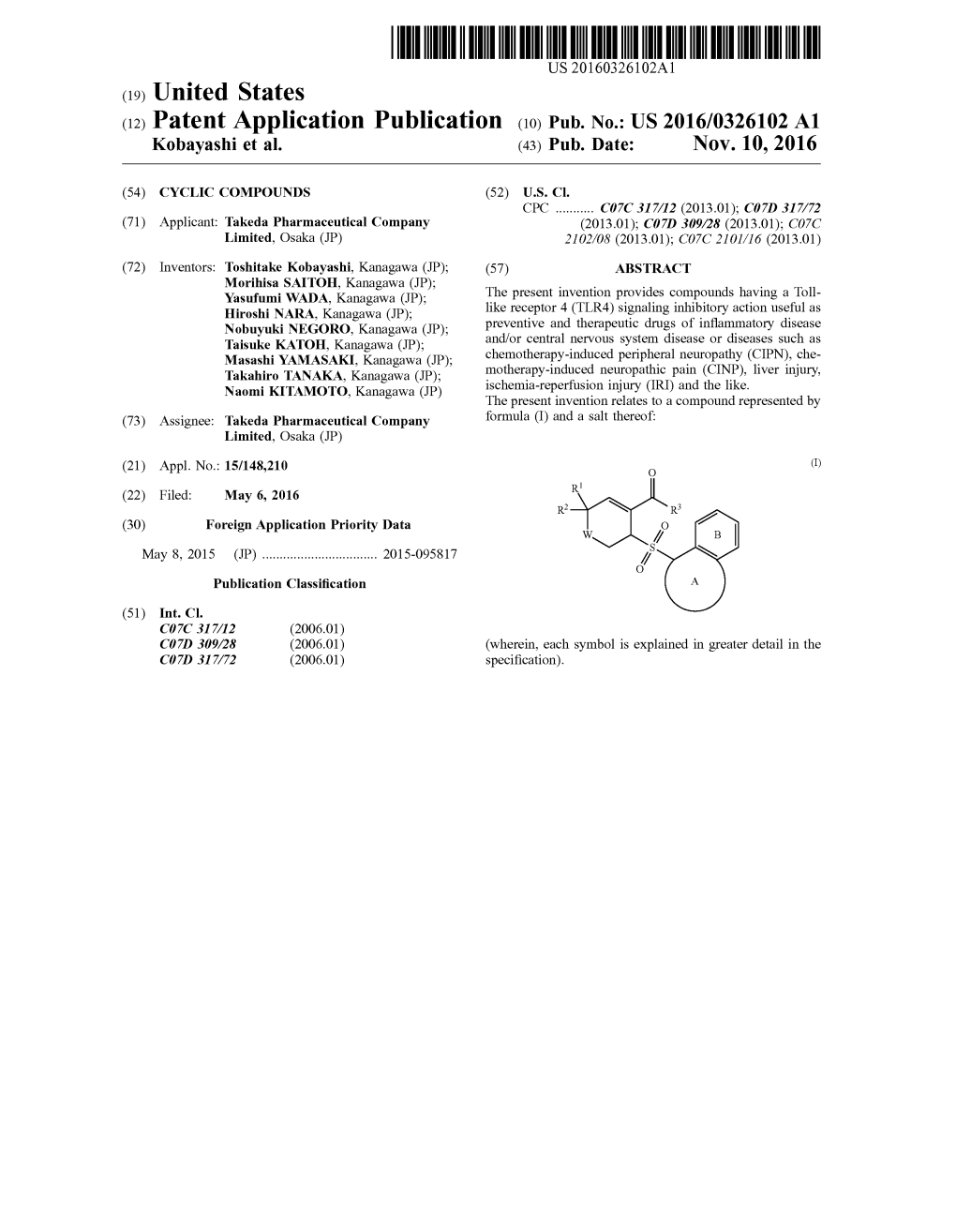
US 2016/0326102 A1 Kobayashi Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synaptic GABAA Receptor Clustering Without the 2 Subunit
The Journal of Neuroscience, July 30, 2014 • 34(31):10219–10233 • 10219 Cellular/Molecular ␥ Synaptic GABAA Receptor Clustering without the 2 Subunit Katalin Kerti-Szigeti, Zoltan Nusser, and X Mark D. Eyre Laboratory of Cellular Neurophysiology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest 1083, Hungary Rapid activation of postsynaptic GABAA receptors (GABAARs) is crucial in many neuronal functions, including the synchronization of neuronal ensembles and controlling the precise timing of action potentials. Although the ␥2 subunit is believed to be essential for the ␥ Ϫ/Ϫ postsynaptic clustering of GABAARs, synaptic currents have been detected in neurons obtained from 2 mice. To determine the role ␥ ␥ of the 2 subunit in synaptic GABAAR enrichment, we performed a spatially and temporally controlled 2 subunit deletion by injecting ␥ 77I Cre-expressing viral vectors into the neocortex of GABAAR 2 lox mice. Whole-cell recordings revealed the presence of miniature IPSCs in Cre ϩ layer 2/3 pyramidal cells (PCs) with unchanged amplitudes and rise times, but significantly prolonged decays. Such slowly decaying currents could be evoked in PCs by action potentials in presynaptic fast-spiking interneurons. Freeze-fracture replica immu- nogold labeling revealed the presence of the ␣1 and 3 subunits in perisomatic synapses of cells that lack the ␥2 subunit. Miniature IPSCs in Cre ϩ PCs were insensitive to low concentrations of flurazepam, providing a pharmacological confirmation of the lack of the ␥2 subunit. Receptors assembled from only ␣ subunits were unlikely because Zn 2ϩ did not block the synaptic currents. Pharmacological experiments indicated that the ␣␥3 receptor, rather than the ␣␦, ␣,or␣␥1 receptors, was responsible for the slowly decaying IPSCs.OurdatademonstratethepresenceofIPSCsandthesynapticenrichmentofthe␣1and3subunitsandsuggestthatthe␥3subunit ␥ is the most likely candidate for clustering GABAARs at synapses in the absence of the 2 subunit. -

Texas Controlled Substances Act
HEALTH AND SAFETY CODE TITLE 6. FOOD, DRUGS, ALCOHOL, AND HAZARDOUS SUBSTANCES SUBTITLE C. SUBSTANCE ABUSE REGULATION AND CRIMES CHAPTER 481. TEXAS CONTROLLED SUBSTANCES ACT SUBCHAPTER A. GENERAL PROVISIONS Sec.A481.001.AASHORT TITLE. This chapter may be cited as the Texas Controlled Substances Act. Acts 1989, 71st Leg., ch. 678, Sec. 1, eff. Sept. 1, 1989. Sec.A481.002.AADEFINITIONS. In this chapter: (1)AA"Administer" means to directly apply a controlled substance by injection, inhalation, ingestion, or other means to the body of a patient or research subject by: (A)AAa practitioner or an agent of the practitioner in the presence of the practitioner; or (B)AAthe patient or research subject at the direction and in the presence of a practitioner. (2)AA"Agent" means an authorized person who acts on behalf of or at the direction of a manufacturer, distributor, or dispenser. The term does not include a common or contract carrier, public warehouseman, or employee of a carrier or warehouseman acting in the usual and lawful course of employment. (3)AA"Commissioner" means the commissioner of state health services or the commissioner 's designee. (4)AA"Controlled premises" means: (A)AAa place where original or other records or documents required under this chapter are kept or are required to be kept; or (B)AAa place, including a factory, warehouse, other establishment, or conveyance, where a person registered under this chapter may lawfully hold, manufacture, distribute, dispense, administer, possess, or otherwise dispose of a controlled substance or other item governed by the federal Controlled Substances Act (21 U.S.C. -

WO 2008/044045 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 17 April 2008 (17.04.2008) WO 2008/044045 Al (51) International Patent Classification: MURRAY, Christopher William [GB/GB]; 436 Cam A61K 31/438 (2006.01) C07D 491/10 (2006.01) bridge Science Park, Milton Road, Cambridge CB4 OQA A61K 31/439 (2006.01) C07D 471/04 (2006.01) (GB). A61K 31/403 (2006.01) C07D 471/10 (2006.01) A61K 31/00 (2006.01) C07D 491/08 (2006.01) (74) Agent: HUTCHINS, Michael, Richard; M. R. Hutchins C07D 209/44 (2006.01) C07D 401/04 (2006.01) & Co., 23 Mount Sion, Tunbridge Wells, Kent TN 1 ITZ C07D 209/08 (2006.01) C07D 401/12 (2006.01) (GB). C07D 215/08 (2006.01) C07D 419/10 (2006.01) (81) Designated States (unless otherwise indicated, for every (21) International Application Number: kind of national protection available): AE, AG, AL, AM, PCT/GB2007/003891 AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, (22) International Filing Date: 12 October 2007 (12.10.2007) ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, (25) Filing Language: English LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, (26) Publication Language: English PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW 60/829.221 12 October 2006 (12.10.2006) US (84) Designated States (unless otherwise indicated, for every (71) Applicant (for all designated States except US): ASTEX kind of regional protection available): ARIPO (BW, GH, THERAPEUTICS LIMITED [GB/GB]; 436 Cambridge GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, Science Park, Milton Road, Cambridge CB4 OQA (GB). -

TSPO in Steroid Neuroendocrinology
231 1 V SELVARAJ and L N TU TSPO in neuroendocrinology 231:1 R1–R30 Review Current status and future perspectives: TSPO in steroid neuroendocrinology Correspondence should be addressed Vimal Selvaraj and Lan N Tu to V Selvaraj Email Department of Animal Science, Cornell University, Ithaca, New York, USA [email protected] Abstract The mitochondrial translocator protein (TSPO), previously known as the peripheral Key Words benzodiazepine receptor (PBR), has received significant attention both as a diagnostic f neurosteroid biomarker and as a therapeutic target for different neuronal disease pathologies. f steroid Recently, its functional basis believed to be mediating mitochondrial cholesterol import f brain for steroid hormone production has been refuted by studies examining both in vivo f spinal cord and in vitro genetic Tspo-deficient models. As a result, there now exists a fundamental f nervous system gap in the understanding of TSPO function in the nervous system, and its putative f mitochondria pharmacology in neurosteroid production. In this review, we discuss several recent f cholesterol Endocrinology findings in steroidogenic cells that are in direct contradiction to previous studies, and f hormone of necessitate a re-examination of the purported role for TSPO in de novo neurosteroid f inflammation biosynthesis. We critically examine the pharmacological effects of different TSPO- Journal binding drugs with particular focus on studies that measure neurosteroid levels. We highlight the basis of key misconceptions regarding TSPO that continue to pervade the literature, and the need for interpretation with caution to avoid negative impacts. We also summarize the emerging perspectives that point to new directions that need to be investigated for understanding the molecular function of TSPO, only after which the Journal of Endocrinology true potential of this therapeutic target in medicine may be realized. -

WO 2013/092791 Al 27 June 2013 (27.06.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/092791 Al 27 June 2013 (27.06.2013) P O P C T (51) International Patent Classification: field Heath, Bishops Stortford, Hertfordshire CM22 7EG C07D 471/04 (2006.01) A61P 9/10 (2006.01) (GB). A61K 31/529 (2006.01) (74) Agent: NICHOL, Maria; Galapagos NV, Chesterford Re (21) International Application Number: search Park, Saffron Walden, Essex CB10 1XL (GB). PCT/EP2012/076275 (81) Designated States (unless otherwise indicated, for every (22) International Filing Date: kind of national protection available): AE, AG, AL, AM, 20 December 2012 (20. 12.2012) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (25) Filing Language: English DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (26) Publication Language: English HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (30) Priority Data: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 61/578,979 22 December 201 1 (22. 12.201 1) US NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (71) Applicant: GALAPAGOS NV [BE/BE]; Industriepark RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, Mechelen Noord, Generaal De Wittelaan L 11/A3, B-2800 TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, Mechelen (BE). -

Modulation of Neurogenesis by PDE Inhibition
(19) & (11) EP 2 377 530 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 19.10.2011 Bulletin 2011/42 A61K 31/40 (2006.01) A61K 45/06 (2006.01) A61K 31/4015 (2006.01) A61K 31/403 (2006.01) (2006.01) (2006.01) (21) Application number: 10013573.0 A61K 31/416 A61K 31/4166 A61K 31/4184 (2006.01) A61K 31/505 (2006.01) (2006.01) (2006.01) (22) Date of filing: 20.10.2006 A61P 25/00 A61K 31/401 (84) Designated Contracting States: • Lorrain, Kym, I. AT BE BG CH CY CZ DE DK EE ES FI FR GB GR San Diego HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI California 92124 (US) SK TR • Pires, Jammieson, C. Chula Vista (30) Priority: 21.10.2005 US 729366 P CA 91911 (US) 21.03.2006 US 784605 P • Treuner, Kai 17.07.2006 US 807594 P San Diego California 92122 (US) (62) Document number(s) of the earlier application(s) in accordance with Art. 76 EPC: (74) Representative: Roques, Sarah Elizabeth 06826395.3 / 1 940 389 J.A. Kemp & Co. 14 South Square (71) Applicant: Braincells, Inc. Gray’s Inn San Diego, CA 92121 (US) London WC1R 5JJ (GB) (72) Inventors: Remarks: • Barlow, Carrolee This application was filed on 12-10-2010 as a Del Mar divisional application to the application mentioned California 92014 (US) under INID code 62. • Carter, Todd, A. San Diego California 92129 (US) (54) Modulation of neurogenesis by PDE inhibition (57) The instant disclosure describes methods for methods based on use of a PDE agent, optionally in com- treating diseases and conditions of the central and pe- bination with one or more other neurogenic agents, to ripheral nervous system by stimulating or increasing neu- stimulate or activate the formation of new nerve cells. -

(12) United States Patent (10) Patent No.: US 8.263,610 B2 Ali Et Al
US008263610B2 (12) United States Patent (10) Patent No.: US 8.263,610 B2 Ali et al. (45) Date of Patent: Sep. 11, 2012 (54) SUBSTITUTED (56) References Cited IMIDAZOLYL-5,6-DIHYDROBENZONISO QUINOLINE COMPOUNDS U.S. PATENT DOCUMENTS 4,522,811 A 6/1985 Eppstein et al. (75) Inventors: Syed M. Ali, North Andoyer, MA (US); 2005/01434424,612,318 AA1 * 9/19866/2005 WintersHudkins et........................ al. 514,293 Mark A. Ashwell, Carlisle, MA (US); 2008.0160028 A1 7/2008 Reicheltet al. Chris Brassard, Somerville, MA (US); Audra Dalton, Revere, MA (US); Anton FOREIGN PATENT DOCUMENTS Filikov, Stoneham, MA (US); Jason EP 104522 A2 4, 1984 Hill, Auburndale, MA (US); Nivedita EP 1238979 A1 9, 2002 Namdev, Westford, MA (US); Rocio E. A. is: Palma, North Andover, MA (US); SU 1626648 A1 9, 1995 Manish Tandon, Framingham, MA WO WO-O 144244 A1 6, 2001 (US); David Vensel, Boston, MA (US); WO WO-2005OO9389 A2 2/2005 JianqiangWang, Acton, MA (US); Neil WO WO-2006010567 A1 2/2006 Westlund, Groton, MA (US) WO WO-2007095628 A1 8, 2007 s s OTHER PUBLICATIONS (73) Assignee: ArCule, Inc., Woburn, MA (US) Diaz-ortizDaz-Ortiz etet al.,al., “Synthesis Tetrahedron, of Pyrazolo56(2000), 3,4-bipyridines pp. 1569-1577. by Cycload (*) b discl h f thi dition Reactions under Microwave Irradiation', Tetrahedron, Notice: Subject to any disclaimer, the term of this 56(11): 1569-1577 (2000). patent is extended or adjusted under 35 International Search Report and Written Opinion for PCT/US2009/ U.S.C. 154(b) by 165 days. 069821, mailed on Apr. -

Synthesis and Pharmacological Activities of Pyrazole Derivatives: a Review
Review Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review Khalid Karrouchi 1,2,3, Smaail Radi 2,*, Youssef Ramli 1, Jamal Taoufik 1, Yahia N. Mabkhot 4,*, Faiz A. Al-aizari 4 and M’hammed Ansar 1 1 Medicinal Chemistry Laboratory, Faculty of Medicine and Pharmacy, Mohammed V University, 10100 Rabat, Morocco; [email protected] (K.K.); [email protected] (Y.R.); [email protected] (J.T.); [email protected] (M.A.) 2 LCAE, Department of Chemistry, Faculty of Sciences, University Mohamed I, 60000 Oujda, Morocco 3 Physicochemical service, Drugs Quality Control Laboratory, Division of Drugs and Pharmacy, Ministry of Health, 10100 Rabat, Morocco 4 Department of Chemistry, Faculty of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia; [email protected] * Correspondence: [email protected] or [email protected] (S.R.); [email protected] (Y.N.M.); Tel.: +212-536-500-601 (S.R.) Received: 22 November 2017; Accepted: 5 January 2018; Published: 12 January 2018 Abstract: Pyrazole and its derivatives are considered a pharmacologically important active scaffold that possesses almost all types of pharmacological activities. The presence of this nucleus in pharmacological agents of diverse therapeutic categories such as celecoxib, a potent anti-inflammatory, the antipsychotic CDPPB, the anti-obesity drug rimonabant, difenamizole, an analgesic, betazole, a H2-receptor agonist and the antidepressant agent fezolamide have proved the pharmacological potential of the pyrazole moiety. Owing to this diversity in the biological field, this nucleus has attracted the attention of many researchers to study its skeleton chemically and biologically. This review highlights the different synthesis methods and the pharmacological properties of pyrazole derivatives. -

September 5-6, 2019 | Athens, Greece Book of Abstracts
6th EFMC Young Medicinal Chemist Symposium September 5-6, 2019 | Athens, Greece Book of Abstracts YMCS19-BoA-Cover.indd 1 30/07/19 09:13 Organising Committees Chairman Dr Emmanouil FOUSTERIS (University of Patras, Patras, Greece) Members Dr David ALKER (David Alker Associates, Birchington, United Kingdom) Prof. Dennis GILLINGHAM (University of Basel, Basel, Switzerland) Dr Kristina GONCHARENKO (SpiroChem AG, Basel, Switzerland) Dr Cassandra LEE FLEMING (University of Gothenburg, Gothenburg, Sweden) Mr Brieuc MATAGNE (LD Organisation, Louvain-la-Neuve, Belgium) Dr Eleni PONTIKI (Aristotele University of Thessaloniki, Thessaloniki, Greece) Dr Matthew TOZER (Consultant, Cambridge, United Kingdom) Prof. Grigorios ZOIDIS (University of Athens, Athens, Greece) Content Welcome 2 Sponsors 3 Programme 5 Keynote Lectures 13 Oral Communications 19 Flash Poster Presentations 39 Posters Presentations 41 List of abstracts 153 List of participants 161 1 Welcome Dear participant, On behalf of the European Federation for Medicinal Chemistry (EFMC) and the Organising Committee, we warmly welcome you to Athens for the 6th edition of the EFMC Young Medicinal Chemist Symposium (EFMC-YMCS). Since the first edition of the EFMC-YMCS in 2014, the symposium has gone from strength to strength with increased participation from EFMC-National Adhering Organisations, and it is now firmly established as the premier forum in Europe for young Medicinal Chemistry and Chemical Biology researchers to promote their science. Our principal aims are: • Creating a network of young European investigators in Medicinal Chemistry and Chemical Biology • Stimulating young European investigators to share their scientific work with peers, and inspiring them to become leaders in their field • Creating competition, excellence and European Champions in Medicinal Chemistry and Chemical Biology This year, more than 170 scientists from 30 nations will gather in Athens for the latest edition. -

Pharmscience Research & Development
Scientific UNITED Group International Conference on PharmScience Research & Development March 04-06, 2019 Venue Paris Marriott Charles de Gaulle Airport Hotel 5 Allee du Verger, Zone Hoteliere Roissy en France, 95700 France Gold Sponsor Publication Partner We are excited to use Whova as our event platform. Attendees please download Whova event app. The event invitation code is: phaqv Floor Plan PARIS CHARLES DE GAULLE Gallery 1 Main Meeting Room WiFi Details Network : Marriott_conf Lunch Password : marriottroissy Gallery 2 Loft 3 Scene 1 Scene 2 Scene 3 Scene 4 Scene 5 Loft 2 Studio Studio Studio Studio Studio Studio Loft 1 1 2 3 Foyer 4 5 6 The Hellenic Society of Medicinal Chemistry (HSMC) supports the publication of the scientific journal ‘Pharmakeftiki’. The publication of the scientific journal ‘Pharmakeftiki’ started in 1987, within the Hellenic Society of Medicinal Chemistry. Later however, it followed an independent pathway as a quarterly edition on pharmaceutical sciences’ topics, always supported by HSMC and recently also by the Hellenic Pharmaceutical Society (HPS). Since 2012 ‘Pharmakeftiki’ is published both as hard copy and on line as an open access journal . The journal ‘Pharmakeftiki’ hosts review and research articles addressing all pharmaceutical topics and relevant scientific areas and promotes information on technological achievements and scientific events. Articles are published after peer-review in both English and Greek language –in the latter case an extended English abstract is required. Articles published in ‘Pharmakeftiki’ are indexed in CHEMICAL ABSTRACTS, EMBASE”, SCOPUS and EBSCO. The electronic version of ‘Pharmakeftiki’ is accessible in the website of HSMC (www.hsmc.gr/en/pharmakeftiki-journal/) and of HPS www.efe.org.gr ***All registered speakers of the Pharma R&D-2019 will be given an opportunity to publish their full-length manuscript as Conference Proceedings in Pharmakeftiki Journal (Scopus Indexed). -

WO 2015/068856 Al 14 May 2015 (14.05.2015) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/068856 Al 14 May 2015 (14.05.2015) W P O P C T (51) International Patent Classification: Yusuke; c/o TAKEDA PHARMACEUTICAL COM C07D 401/14 (2006.01) C07D 409/14 (2006.01) PANY LIMITED, 26-1, Muraoka-Higashi 2-chome, C07D 413/14 (2006.01) C07D 417/14 (2006.01) Fujisawa-shi, Kanagawa, 25 10012 (JP). SHIOKAWA, A61K 31/4155 (2006.01) C07D 487/04 (2006.01) Zenyu; c/o TAKEDA PHARMACEUTICAL COMPANY C07D 401/04 (2006.01) C07D 487/10 (2006.01) LIMITED, 26-1, Muraoka-Higashi 2-chome, Fujisawa-shi, C07D 403/14 (2006.01) A61P 37/00 (2006.01) Kanagawa, 2510012 (JP). SHIBUYA, Akito; c/o TAKE DA PHARMACEUTICAL COMPANY LIMITED, 26-1, (21) International Application Number: Muraoka-Higashi 2-chome, Fujisawa-shi, Kanagawa, PCT/JP20 14/080005 25 10012 (JP). SASAKI, Yusuke; c/o TAKEDA PHAR (22) International Filing Date: MACEUTICAL COMPANY LIMITED, 26-1, Muraoka- 5 November 20 14 (05 .11.20 14) Higashi 2-chome, Fujisawa-shi, Kanagawa, 2510012 (JP). GIBSON, Tony; c/o TAKEDA CALIFORNIA, INC., (25) Filing Language: English 10410 Science Center Drive, San Diego, California, 92121 (26) Publication Language: English (US). TAKAGI, Terufumi; c/o TAKEDA PHARMA CEUTICAL COMPANY LIMITED, 26-1, Muraoka-Hi (30) Priority Data: gashi 2-chome, Fujisawa-shi, Kanagawa, 25 10012 (JP). 2013-232571 8 November 2013 (08. 11.2013) JP 2014- 128562 23 June 2014 (23.06.2014) JP (74) Agent: TAKASHIMA, Hajime; Meiji Yasuda Seimei Osaka Midosuji Bldg., 1-1, Fushimimachi 4-chome, Chuo- (71) Applicant: TAKEDA PHARMACEUTICAL COM¬ ku, Osaka-shi, Osaka, 5410044 (JP). -

Barbiturate Receptor Sites Are Coupled to Benzodiazepine Receptors
Proc. Natl. Acad. Sci. USA Vol. 77, No. 12, pp. 7468-7472, December 1980 Neurobiology Barbiturate receptor sites are coupled to benzodiazepine receptors (anesthetic/anticonvulsant/'y-aminobutyric acid receptor/Cl channel) FREDRIK LEEB-LUNDBERG, ADELE SNOWMAN, AND RICHARD W. OLSEN Division of Biomedical Sciences and Department of Biochemistry, University of California, Riverside, California 92521 Communicated by George A. Zentmyer, August 11, 1980 ABSTRACT Barbiturates enhance the binding of [3HJdi- radioactive picrotoxinin (a-[3H]dihydropicrotoxinin, [3H]DHP) azepam to benzodiazepine receptor sites in rat brain. This effect occurs at pharmacologically relevant concentrations of barbi- (9, 10). [3H]DHP binding is inhibited by micromolar concen- turates, and the relative activity of a series of compounds cor- trations of depressant barbiturates (9, 10), submicromolar relates highly with anesthetic activity of the barbiturates and concentrations of excitatory barbiturates (9, 10), and other with their ability to enhance postsynaptic inhibitory responses convulsant drugs that can block GABA synapses (10, 11). to the neurotransmitter 'y-aminobutyric acid. Barbiturate en- Another class of central nervous system-depressant drugs that hancement of benzodiazepine binding is stereospecific, with may act at least in part by enhancement of GABAergic inhib- the more active anesthetic isomers of Nl-methylbarbiturates being also more active than their stereoisomers in enhancing itory synaptic transmission (5) are the benzodiazepines (BZ), benzodiazepine binding. The active barbiturates produce a such as diazepam. These compounds bind to high-affinity re- reversible enhancement in the affinity of specific benzodiaze- ceptor sites in the central nervous system with a chemical pine binding with no effect on the number of binding sites.