Synthesis and Pharmacological Activities of Pyrazole Derivatives: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synaptic GABAA Receptor Clustering Without the 2 Subunit
The Journal of Neuroscience, July 30, 2014 • 34(31):10219–10233 • 10219 Cellular/Molecular ␥ Synaptic GABAA Receptor Clustering without the 2 Subunit Katalin Kerti-Szigeti, Zoltan Nusser, and X Mark D. Eyre Laboratory of Cellular Neurophysiology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest 1083, Hungary Rapid activation of postsynaptic GABAA receptors (GABAARs) is crucial in many neuronal functions, including the synchronization of neuronal ensembles and controlling the precise timing of action potentials. Although the ␥2 subunit is believed to be essential for the ␥ Ϫ/Ϫ postsynaptic clustering of GABAARs, synaptic currents have been detected in neurons obtained from 2 mice. To determine the role ␥ ␥ of the 2 subunit in synaptic GABAAR enrichment, we performed a spatially and temporally controlled 2 subunit deletion by injecting ␥ 77I Cre-expressing viral vectors into the neocortex of GABAAR 2 lox mice. Whole-cell recordings revealed the presence of miniature IPSCs in Cre ϩ layer 2/3 pyramidal cells (PCs) with unchanged amplitudes and rise times, but significantly prolonged decays. Such slowly decaying currents could be evoked in PCs by action potentials in presynaptic fast-spiking interneurons. Freeze-fracture replica immu- nogold labeling revealed the presence of the ␣1 and 3 subunits in perisomatic synapses of cells that lack the ␥2 subunit. Miniature IPSCs in Cre ϩ PCs were insensitive to low concentrations of flurazepam, providing a pharmacological confirmation of the lack of the ␥2 subunit. Receptors assembled from only ␣ subunits were unlikely because Zn 2ϩ did not block the synaptic currents. Pharmacological experiments indicated that the ␣␥3 receptor, rather than the ␣␦, ␣,or␣␥1 receptors, was responsible for the slowly decaying IPSCs.OurdatademonstratethepresenceofIPSCsandthesynapticenrichmentofthe␣1and3subunitsandsuggestthatthe␥3subunit ␥ is the most likely candidate for clustering GABAARs at synapses in the absence of the 2 subunit. -

(12) United States Patent (10) Patent No.: US 8,361,492 B2 Tauber Et Al
USOO8361492B2 (12) United States Patent (10) Patent No.: US 8,361,492 B2 Tauber et al. (45) Date of Patent: *Jan. 29, 2013 (54) DRUG DELIVERY SYSTEMAND METHODS (56) References Cited OF USE U.S. PATENT DOCUMENTS (75) Inventors: Shachar Tauber, Ozark, MO (US); 2003/0017208 A1* 1/2003 Ignatious et al. ............. 424/486 Randall Fuerst, Orangevale, CA (US); 2003/01931 18 A1 10/2003 Bango et al. 2003/0215624 A1 1 1/2003 Layman et al. Keela Davis, Springfield, MO (US); Lyle 2003/0232287 A1 12/2003 Bango Bowman, Pleasanton, CA (US); Gary 2004/0018226 A1 1/2004 Winek et al. Wnek, Cleveland, OH (US); Joseph J. 2005, OO67287 A1 3/2005 Fuerst et al. Bango, Jr., New Haven, CT (US) 2006/0085063 A1* 4/2006 Shastri et al. ................ 623, 141 2006/0171991 A1 8/2006 Bango 2006/0246113 A1 11/2006 Griffith et al. (73) Assignee: Ocugenics, LLC, Orangevale, CA (US) 2008.0002149 A1 1/2008 Fritsch et al. 2009/0217849 A1 9, 2009 Eastin et al. (*) Notice: Subject to any disclaimer, the term of this 2009, 0238858 A1 9, 2009 Kohnet al. patent is extended or adjusted under 35 OTHER PUBLICATIONS U.S.C. 154(b) by 493 days. Office Action issued in related U.S. Appl. No. 12/416,802, dated Sep. This patent is Subject to a terminal dis 17, 2010, 11 pages. claimer. International Search Report and Written Opinion issued in PCT/ US2010/029126, dated Jan. 14, 2011, 11 pages. Kenawy, E.R. et al. “Controlled Release of Ketoprofen from (21) Appl. No.: 12/490,972 electrospun poly(vinyl alcohol) nanofibers' Materials Science & Engineering A 459, pp. -

194 Recent Advances in the Synthesis of New Pyrazole Derivatives
194 RECENT ADVANCES IN THE SYNTHESIS OF NEW PYRAZOLE DERIVATIVES DOI: http://dx.medra.org/ 10.17374/targets.2019.22.194 Juan - Carlos Castillo a,b , Jaime Portilla a * a Bioorganic Compounds Research Group, Department of Chemistry, Universi dad de los Andes, Carrera 1 No. 18A - 10, Bogotá, Colombia b Grupo de Cat álisis, E scuela de Ciencias Químicas, Universidad Pedagógica y Tecnológica de Colombia UPTC, Av enida Central del Norte, Tunja, Colombia (e - mail : [email protected] ) Abstract. Pyrazoles have attracted great attention in organic and medicinal chemistry, due to their proven utility as synthetic intermediates for the preparation of diverse bioactive compounds, of coordination complexes, as well as in the design of functional materials. Consequently , the synthesis of functionalized pyrazoles is an important focus of research for synthetic organic chemists. Likewise, fuse d pyrazoles such as pyrazolo[1,5 - a]pyrimidines and pyrazolo[3,4 - b]pyridines have been widely studied due to their varied biological and physicochemical applications based on the important electronic properties of th ese N - heterocycles. Therefore, the preparation of these fused heterocycles and of their functionalized derivatives is of notable interest to both uncover novel derivatives and explore new applications. Several methods have been described in the literature for the synthesis of pyrazoles and of their fused systems in recent years , which mainly involve classi cal cyclocondensation reactions , some of these are presented in th is contribution. Contents 1. Introduction 2. Functionalized pyrazoles 2.1. Aminopyrazoles 2.2. Formylpyrazoles 3. Fused pyrazoles 3.1. Pyrazolo[1,5 - a ]pyrimidines 3.2. Pyrazolo[3,4 - b ]pyridines 3.3. -

Development of Novel Peptide Nucleic Acids A
b-Dicarbonyl compounds Compounds having two carbonyl groups separated by an intervening carbon atom are called b-dicarbonyl compounds, and these compounds are highly versatile reagents for organic synthesis. O O O O C C C R C C C OR' b b b-Dicarbonyl system b-Keto ester ** The pKa for such a proton is in the range 9-11, acidic enough to be removed easily by an alkoxide base to form an enolate. O O O H O OR C C C C C.. C + HOR H enolate anion pKa = 9-11 .. .. .. .. .. .. O . O . O O . O O C C C .. C C C R C OR' R C OR' R C OR' H H H Resonance structure of the anion of a b-keto ester Synthesis of b-keto ester (Claisen condensation) O O O NaOC H 2 2 5 CH3COC2H5 CH3CC..HCOC2H5 + C2H5OH + Na (removed by distillation) Sodiumacetoacetic ester HCl O O CH3CCH2COC2H5 Ethyl acetoacetate (acetoacetic ester) (76%) O O O O (1) NaOC2H5 R CH C + H CHC R CH2C CHCOC H + C H OH 2 OC2H5 OC2H5 + 2 5 2 5 (2) H3O R R (R may also be H) b-Keto ester Mechanism Step1 .. O O . .. + . OHC H + C H OH R CHC OC2H5 .. 2 5 RC.. H C OC2H5 2 5 H .. O . RCH C OC2H5 Step 2 .. .. .. .. O . O O O . RCH2C + . RCH2C CH C OC2H5 HC C OC2H5 . C H O . OC2H5 R 2 5 .. R .. O . O .. + . OC2H5 RCH2C CH C OC2H5 .. R Step 3 .. .. O . O H O O . -

WO 2012/044761 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date _ . 5 April 2012 (05.04.2012) WO 2012/044761 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 47/48 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US201 1/053876 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 29 September 201 1 (29.09.201 1) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, (26) Publication Langi English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW. 12/893,344 29 September 2010 (29.09.2010) US (84) Designated States (unless otherwise indicated, for every (71) Applicant (for all designated States except US): UNI¬ kind of regional protection available): ARIPO (BW, GH, VERSITY OF NORTH CAROLINA AT WILMING¬ GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, TON [US/US]; 601 South College Road, Wilmington, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, NC 28403 (US). -

Aniline and Aniline Hydrochloride
SOME AROMATIC AMINES AND RELATED COMPOUNDS VOLUME 127 This publication represents the views and expert opinions of an IARC Working Group on the Identification of Carcinogenic Hazards to Humans, which met remotely, 25 May–12 June 2020 LYON, FRANCE - 2021 IARC MONOGRAPHS ON THE IDENTIFICATION OF CARCINOGENIC HAZARDS TO HUMANS ANILINE AND ANILINE HYDROCHLORIDE 1. Exposure Characterization 1.1.2 Structural and molecular formulae, and relative molecular mass 1.1 Identification of the agent (a) Aniline 1.1.1 Nomenclature NH2 (a) Aniline Chem. Abstr. Serv. Reg. No.: 62-53-3 EC No.: 200-539-3 Molecular formula: C H N IUPAC systematic name: aniline 6 7 Relative molecular mass: 93.13 (NCBI, 2020a). Synonyms and abbreviations: benzenamine; phenylamine; aminobenzene; aminophen; (b) Aniline hydrochloride aniline oil. NH2 (b) Aniline hydrochloride Chem. Abstr. Serv. Reg. No.: 142-04-1 EC No.: 205-519-8 HCl IUPAC systematic name: aniline hydro - Molecular formula: C6H8ClN chloride Relative molecular mass: 129.59 (NCBI, Synonyms: aniline chloride; anilinium chlo- 2020b). ride; benzenamine hydrochloride; aniline. HCl; phenylamine hydrochloride; phenylam- monium chloride. 1.1.3 Chemical and physical properties of the pure substance Aniline is a basic compound and will undergo acid–base reactions. Aniline and its hydrochlo- ride salt will achieve a pH-dependent acid–base equilibrium in the body. 109 IARC MONOGRAPHS – 127 (a) Aniline Octanol/water partition coefficient (P): log Kow, 0.936, predicted median (US EPA, 2020b) Description: aniline appears as a yellowish Conversion factor: 1 ppm = 5.3 mg/m3 [calcu- to brownish oily liquid with a musty fishy lated from: mg/m3 = (relative molecular odour (NCBI, 2020a), detectable at 1 ppm 3 mass/24.45) × ppm, assuming temperature [3.81 mg/m ] (European Commission, 2016; (25 °C) and pressure (101 kPa)]. -

Lonza's Chemical Network Diketene and HCN Derivatives, Heterocycles
Lonza’s chemical network Diketene and HCN derivatives, heterocycles and basic chemicals catalog Pharma&Biotech Nutrition Agriculture MaterialsScience PersonalCare Diketene and HCN derivatives, heterocycles and basic chemicals catalog Content About Lonza 3 Introduction 4 Diketene / ketene derivatives 6 Esters 6 Arylides 7 Alkylamides 8 Pyrazolones 9 Dehydroacetic acid 9 Lonzamon monomers 10 Other diketene derivatives 10 HCN derivatives 12 Heterocycles 14 Basic chemicals 16 Others 17 Alphabetical index 18 About Lonza Lonza is the global leader in the production and support of active phar- From 1897 to the present day combining Swiss tradition with global maceutical ingredients both chemically and biotechnologically. Biophar- experience, the company has had an enterprising character, adapting maceuticals are one of the key growth drivers of the pharmaceutical and its offerings and services to the needs of customers and to changing biotechnology industries. technologies. Throughout our history, we have maintained a strong culture of performance, results and dependability that is valued by all Lonza has strong capabilities in large and small molecules, peptides, of our customers. amino acids and niche bioproducts which play an important role in the development of novel medicines and healthcare products. In addition, Lonza’s cracker in Visp is the back bone of a comprehensive fully back- Lonza is a leader in cell-based research, endotoxin detection and cell ward integrated chemical network. Our product portfolio consists of therapy manufacturing. Furthermore, the company is a leading provider HCN- and diketene derivatives as well as basic chemicals which are key of chemical and biotech ingredients to the nutrition, hygiene, preserva- raw materials and intermediates for many sophisticated applications. -

Synthetic Turf Scientific Advisory Panel Meeting Materials
California Environmental Protection Agency Office of Environmental Health Hazard Assessment Synthetic Turf Study Synthetic Turf Scientific Advisory Panel Meeting May 31, 2019 MEETING MATERIALS THIS PAGE LEFT BLANK INTENTIONALLY Office of Environmental Health Hazard Assessment California Environmental Protection Agency Agenda Synthetic Turf Scientific Advisory Panel Meeting May 31, 2019, 9:30 a.m. – 4:00 p.m. 1001 I Street, CalEPA Headquarters Building, Sacramento Byron Sher Auditorium The agenda for this meeting is given below. The order of items on the agenda is provided for general reference only. The order in which items are taken up by the Panel is subject to change. 1. Welcome and Opening Remarks 2. Synthetic Turf and Playground Studies Overview 4. Synthetic Turf Field Exposure Model Exposure Equations Exposure Parameters 3. Non-Targeted Chemical Analysis Volatile Organics on Synthetic Turf Fields Non-Polar Organics Constituents in Crumb Rubber Polar Organic Constituents in Crumb Rubber 5. Public Comments: For members of the public attending in-person: Comments will be limited to three minutes per commenter. For members of the public attending via the internet: Comments may be sent via email to [email protected]. Email comments will be read aloud, up to three minutes each, by staff of OEHHA during the public comment period, as time allows. 6. Further Panel Discussion and Closing Remarks 7. Wrap Up and Adjournment Agenda Synthetic Turf Advisory Panel Meeting May 31, 2019 THIS PAGE LEFT BLANK INTENTIONALLY Office of Environmental Health Hazard Assessment California Environmental Protection Agency DRAFT for Discussion at May 2019 SAP Meeting. Table of Contents Synthetic Turf and Playground Studies Overview May 2019 Update ..... -

Acetoacetic Ester Synthesis
Programme: B.Sc. B.ed. (Integrated) Course: ORGANIC CHEMISTRY- III Semester: VI Code: CHE-352 Topic: ETHYLACETOACETATEE Date- 07/04/2020 y Only PPe Dr. Angad Kumar Singh ForF Department of Chemistry, Central University of South Bihar, Gaya (Bihar) Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Ethylacetoacetate The Claisen Condensation between esters containing - hdhydrogens, promotdted byabase such as sodium ethoxy ide, toproduce a !- ketoester. One equiv serves as the nucleophilele (enolate)(eno and the other is OnlyO the electrophile which undergoes additiontion andae elimination. The use of stronger bases, e.g. sodium amide or sodiumsodUse hydride instead of sodium ethoxide, often increases the yield.d. al onal Ethylacetoacetate Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mechanism of the Claisen Condensation The reaction is driven to product by the final deprotonation step. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mixed Claisen Condensation Like mixed aldol reactions, mixed Claisen condensations are useful if differences in reactivity exist betweenn they two esters as for example when one of the esters has no -hydrogenydrogOnlyO . Examples of such esters are: e Use ers excess Note: These materials are only for classroom teaching purpose at Central University of South Bihar. -
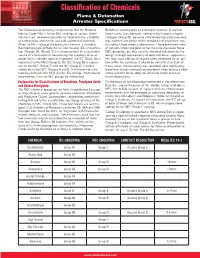
Classification of Chemicals
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

Texas Controlled Substances Act
HEALTH AND SAFETY CODE TITLE 6. FOOD, DRUGS, ALCOHOL, AND HAZARDOUS SUBSTANCES SUBTITLE C. SUBSTANCE ABUSE REGULATION AND CRIMES CHAPTER 481. TEXAS CONTROLLED SUBSTANCES ACT SUBCHAPTER A. GENERAL PROVISIONS Sec.A481.001.AASHORT TITLE. This chapter may be cited as the Texas Controlled Substances Act. Acts 1989, 71st Leg., ch. 678, Sec. 1, eff. Sept. 1, 1989. Sec.A481.002.AADEFINITIONS. In this chapter: (1)AA"Administer" means to directly apply a controlled substance by injection, inhalation, ingestion, or other means to the body of a patient or research subject by: (A)AAa practitioner or an agent of the practitioner in the presence of the practitioner; or (B)AAthe patient or research subject at the direction and in the presence of a practitioner. (2)AA"Agent" means an authorized person who acts on behalf of or at the direction of a manufacturer, distributor, or dispenser. The term does not include a common or contract carrier, public warehouseman, or employee of a carrier or warehouseman acting in the usual and lawful course of employment. (3)AA"Commissioner" means the commissioner of state health services or the commissioner 's designee. (4)AA"Controlled premises" means: (A)AAa place where original or other records or documents required under this chapter are kept or are required to be kept; or (B)AAa place, including a factory, warehouse, other establishment, or conveyance, where a person registered under this chapter may lawfully hold, manufacture, distribute, dispense, administer, possess, or otherwise dispose of a controlled substance or other item governed by the federal Controlled Substances Act (21 U.S.C. -

Synthesis of Pyrazoles Containing Benzofuran and Trifluoromethyl Moieties As Possible Anti-Inflammatory and Analgesic Agents
Z. Naturforsch. 2015; 70(7)b: 519–526 Awatef A. Farag, Mohamed F. El Shehry, Samir Y. Abbas*, Safaa N. Abd-Alrahman, Abeer A. Atrees, Hiaat Z. Al-basheer and Yousry A. Ammar Synthesis of pyrazoles containing benzofuran and trifluoromethyl moieties as possible anti-inflammatory and analgesic agents DOI 10.1515/znb-2015-0009 and anti-inflammatory drugs present a wide range of prob- Received January 9, 2015; accepted February 4, 2015 lems such as efficacy and undesired effects including gas- trointestinal tract (GIT) disorders and other unwanted effects. This situation highlights the need for novel, safe and Abstract: Searching for new anti-inflammatory and anal- effective analgesic and anti-inflammatory compounds [1–3]. gesic agents, we have prepared a series of novel pyrazoles Since the first pyrazolin-5-one was prepared by Knorr containing benzofuran and trifluoromethyl moieties. The [4] in 1883, many papers have reported on the anti-inflam- pyrazole derivatives have been synthesized via two routes matory, analgesic and antipyretic evaluation of several starting from 5-(3-(trifluoromethyl)phenyl azo) salicylal- pyrazoles, pyrazolin-3-ones and pyrazolidine-3,5-diones dehyde. The first route involved the synthesis of 2-acetylb- [5–9]. Many of these derivatives such as phenylbutazone, enzofuran and then treatment with aldehydes to afford febrazone, feclobuzone, mefobutazone, suxibuzone and the corresponding chalcones. The cyclization of the latter ramifenazone have found their clinical application as chalcones with hydrazine hydrate led to the formation of nonsteroidal anti-inflammatory drugs (NSAIDs) [10]. new pyrazoline derivatives. The second route involved the The benzofuran derivatives have attracted due to their synthesis of benzofuran-2-carbohydrazide and then treat- biological activities and potential application as pharma- ment with formylpyrazoles, chalcones and ketene dith- cological agents [11].