Determination of Solvent Effects on Ketoðenol Equilibria of 1,3-Dicarbonyl Compounds Using NMR: Revisiting a Classic Physical C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety
Int. J. Mol. Sci. 2014, 15, 1237-1254; doi:10.3390/ijms15011237 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety Elham S. Darwish *, Azza M. Abdel Fattah, Fawzy A. Attaby and Oqba N. Al-Shayea Department of Chemistry, Faculty of Science, Cairo University, Giza 12613, Egypt; E-Mails: [email protected] (A.M.A.F.); [email protected] (F.A.A.); [email protected] (O.N.A.-S.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +20-100-588-1771; Fax: +202-570-8480. Received: 10 November 2013; in revised form: 10 January 2014 / Accepted: 13 January 2014 / Published: 17 January 2014 Abstract: This study aimed for the synthesis of new heterocyclic compounds incorporating sulfamoyl moiety suitable for use as antimicrobial agents via a versatile, readily accessible N-[4-(aminosulfonyl)phenyl]-2-cyanoacetamide (3). The 2-pyridone derivatives were obtained via reaction of cyanoacetamide with acetylacetone or arylidenes malononitrile. Cycloaddition reaction of cyanoacetamide with salicyaldehyde furnished chromene derivatives. Diazotization of 3 with the desired diazonium chloride gave the hydrazone derivatives 13a–e. Also, the reactivity of the hydrazone towards hydrazine hydrate to give Pyrazole derivatives was studied. In addition, treatment of 3 with elemental sulfur and phenyl isothiocyanate or malononitrile furnished thiazole and thiophene derivatives respectively. Reaction of 3 with phenyl isothiocyanate and KOH in DMF afforded the intermediate salt 17 which reacted in situ with 3-(2-bromoacetyl)-2H-chromen-2-one and methyl iodide afforded the thiazole and ketene N,S-acetal derivatives respectively. -

Feiii, Cuii and Znii Complexes of the Rigid 9-Oxido-Phenalenone Ligand—Spectroscopy, Electrochemistry, and Cytotoxic Properties
International Journal of Molecular Sciences Article FeIII, CuII and ZnII Complexes of the Rigid 9-Oxido-phenalenone Ligand—Spectroscopy, Electrochemistry, and Cytotoxic Properties Katharina Butsch 1, Alexander Haseloer 1 , Simon Schmitz 1, Ingo Ott 2, Julia Schur 2 and Axel Klein 1,* 1 Department für Chemie, Institut für Anorganische Chemie, Universität zu Köln, Greinstraße 6, D-50939 Köln, Germany; [email protected] (K.B.); [email protected] (A.H.); [email protected] (S.S.) 2 Institute of Medicinal and Pharmaceutical Chemistry, Technische Universität Braunschweig, Beethovenstrasse 55, D-38106 Braunschweig, Germany; [email protected] (I.O.); [email protected] (J.S.) * Correspondence: [email protected] Abstract: The three complexes [Fe(opo)3], [Cu(opo)2], and [Zn(opo)2] containing the non-innocent anionic ligand opo− (opo− = 9-oxido-phenalenone, Hopo = 9-hydroxyphenalonone) were synthe- 1 sised from the corresponding acetylacetonates. [Zn(opo)2] was characterised using H nuclear magnetic resonance (NMR) spectroscopy, the paramagnetic [Fe(opo)3] and [Cu(opo)2] by electron paramagnetic resonance (EPR) spectroscopy. While the EPR spectra of [Cu(opo)2] and [Cu(acac)2] in dimethylformamide (DMF) solution are very similar, a rather narrow spectrum was observed for [Fe(opo)3] in tetrahydrofuran (THF) solution in contrast to the very broad spectrum of [Fe(acac)3] in − Citation: Butsch, K.; Haseloer, A.; THF (Hacac = acetylacetone, 2,4-pentanedione; acac = acetylacetonate). The narrow, completely Schmitz, S.; Ott, I.; Schur, J.; Klein, A. isotropic signal of [Fe(opo)3] disagrees with a metal-centred S = 5/2 spin system that is observed FeIII, CuII and ZnII Complexes of the in the solid state. -
Ochem ACS Review 18 Enols and Enolates
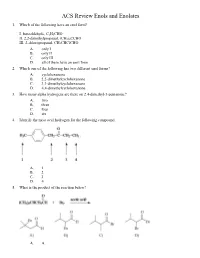
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -

Development of Novel Peptide Nucleic Acids A
b-Dicarbonyl compounds Compounds having two carbonyl groups separated by an intervening carbon atom are called b-dicarbonyl compounds, and these compounds are highly versatile reagents for organic synthesis. O O O O C C C R C C C OR' b b b-Dicarbonyl system b-Keto ester ** The pKa for such a proton is in the range 9-11, acidic enough to be removed easily by an alkoxide base to form an enolate. O O O H O OR C C C C C.. C + HOR H enolate anion pKa = 9-11 .. .. .. .. .. .. O . O . O O . O O C C C .. C C C R C OR' R C OR' R C OR' H H H Resonance structure of the anion of a b-keto ester Synthesis of b-keto ester (Claisen condensation) O O O NaOC H 2 2 5 CH3COC2H5 CH3CC..HCOC2H5 + C2H5OH + Na (removed by distillation) Sodiumacetoacetic ester HCl O O CH3CCH2COC2H5 Ethyl acetoacetate (acetoacetic ester) (76%) O O O O (1) NaOC2H5 R CH C + H CHC R CH2C CHCOC H + C H OH 2 OC2H5 OC2H5 + 2 5 2 5 (2) H3O R R (R may also be H) b-Keto ester Mechanism Step1 .. O O . .. + . OHC H + C H OH R CHC OC2H5 .. 2 5 RC.. H C OC2H5 2 5 H .. O . RCH C OC2H5 Step 2 .. .. .. .. O . O O O . RCH2C + . RCH2C CH C OC2H5 HC C OC2H5 . C H O . OC2H5 R 2 5 .. R .. O . O .. + . OC2H5 RCH2C CH C OC2H5 .. R Step 3 .. .. O . O H O O . -

Lonza's Chemical Network Diketene and HCN Derivatives, Heterocycles
Lonza’s chemical network Diketene and HCN derivatives, heterocycles and basic chemicals catalog Pharma&Biotech Nutrition Agriculture MaterialsScience PersonalCare Diketene and HCN derivatives, heterocycles and basic chemicals catalog Content About Lonza 3 Introduction 4 Diketene / ketene derivatives 6 Esters 6 Arylides 7 Alkylamides 8 Pyrazolones 9 Dehydroacetic acid 9 Lonzamon monomers 10 Other diketene derivatives 10 HCN derivatives 12 Heterocycles 14 Basic chemicals 16 Others 17 Alphabetical index 18 About Lonza Lonza is the global leader in the production and support of active phar- From 1897 to the present day combining Swiss tradition with global maceutical ingredients both chemically and biotechnologically. Biophar- experience, the company has had an enterprising character, adapting maceuticals are one of the key growth drivers of the pharmaceutical and its offerings and services to the needs of customers and to changing biotechnology industries. technologies. Throughout our history, we have maintained a strong culture of performance, results and dependability that is valued by all Lonza has strong capabilities in large and small molecules, peptides, of our customers. amino acids and niche bioproducts which play an important role in the development of novel medicines and healthcare products. In addition, Lonza’s cracker in Visp is the back bone of a comprehensive fully back- Lonza is a leader in cell-based research, endotoxin detection and cell ward integrated chemical network. Our product portfolio consists of therapy manufacturing. Furthermore, the company is a leading provider HCN- and diketene derivatives as well as basic chemicals which are key of chemical and biotech ingredients to the nutrition, hygiene, preserva- raw materials and intermediates for many sophisticated applications. -

Glycosyl Barbiturate Ligands of Bacterial Lectins: from Monomer Design to Glycoclusters and Glycopolymers François Portier, Anne Imberty, Sami Halila
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archive Ouverte en Sciences de l'Information et de la Communication Expeditious Synthesis of C -Glycosyl Barbiturate Ligands of Bacterial Lectins: From Monomer Design to Glycoclusters and Glycopolymers François Portier, Anne Imberty, Sami Halila To cite this version: François Portier, Anne Imberty, Sami Halila. Expeditious Synthesis of C -Glycosyl Barbiturate Lig- ands of Bacterial Lectins: From Monomer Design to Glycoclusters and Glycopolymers. Bioconjugate Chemistry, American Chemical Society, 2019, 30 (3), pp.647-656. 10.1021/acs.bioconjchem.8b00847. hal-02322076 HAL Id: hal-02322076 https://hal.archives-ouvertes.fr/hal-02322076 Submitted on 4 Aug 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. EXPEDITIOUS SYNTHESIS OF C‐GLYCOSYL BARBITURATE LIGANDS OF BACTERIAL LECTINS: FROM MONOMER DESIGN TO GLYCOCLUSTERS AND GLYCOPOLYMERS François Portier, Anne Imberty and Sami Halila* Univ. Grenoble Alpes, CNRS, CERMAV, 38000 Grenoble, France. E‐mail: [email protected]; Fax: +33 4 76 54 72 03; Tel : +33 4 76 03 76 66 ABSTRACT The approach developed here offers a straightforward and efficient access to ‐C‐glycosyl barbiturates ligands, spanning from glycomimetics to multivalent C‐neoglycoconjugates, with the aim of deciphering structural parameters impacting the binding to pathogenic lectins. -

Acetoacetic Ester Synthesis
Programme: B.Sc. B.ed. (Integrated) Course: ORGANIC CHEMISTRY- III Semester: VI Code: CHE-352 Topic: ETHYLACETOACETATEE Date- 07/04/2020 y Only PPe Dr. Angad Kumar Singh ForF Department of Chemistry, Central University of South Bihar, Gaya (Bihar) Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Ethylacetoacetate The Claisen Condensation between esters containing - hdhydrogens, promotdted byabase such as sodium ethoxy ide, toproduce a !- ketoester. One equiv serves as the nucleophilele (enolate)(eno and the other is OnlyO the electrophile which undergoes additiontion andae elimination. The use of stronger bases, e.g. sodium amide or sodiumsodUse hydride instead of sodium ethoxide, often increases the yield.d. al onal Ethylacetoacetate Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mechanism of the Claisen Condensation The reaction is driven to product by the final deprotonation step. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mixed Claisen Condensation Like mixed aldol reactions, mixed Claisen condensations are useful if differences in reactivity exist betweenn they two esters as for example when one of the esters has no -hydrogenydrogOnlyO . Examples of such esters are: e Use ers excess Note: These materials are only for classroom teaching purpose at Central University of South Bihar. -

Classification of Chemicals
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

Regioselective Addition of 1,3-Dicarbonyl Dianion to N-Sulfonyl Aldimines: an Expedient Route to N-Sulfonyl Piperidines and N-Sulfonyl Azetidines
Tetrahedron 63 (2007) 4779–4787 Regioselective addition of 1,3-dicarbonyl dianion to N-sulfonyl aldimines: an expedient route to N-sulfonyl piperidines and N-sulfonyl azetidines Manas K. Ghorai,* Amit Kumar and Sandipan Halder Department of Chemistry, Indian Institute of Technology, Kanpur 208 016, India Received 26 June 2006; revised 23 February 2007; accepted 15 March 2007 Available online 18 March 2007 Abstract—A simple route for the synthesis of d-amino-b-keto esters and d-amino-b-diketones is reported. This involves regioselective addition of 1,3-dianions derived from ethyl acetoacetate and acetyl acetone to N-sulfonyl aldimines. The d-amino-b-keto ester derivatives were further converted into the corresponding N-sulfonyl piperidines and N-sulfonyl azetidines. Ó 2007 Elsevier Ltd. All rights reserved. 1. Introduction In continuation of our research on enolate chemistry and also supported by the related literature on the reactivity of 1,3-di- 1,3-Dicarbonyl dianions have been used extensively in or- carbonyl dianions, it was envisaged that dianions derived ganic synthesis for building ring systems. The anion on the from suitable precursors could be regioselectively added to terminal carbon of the dianion can be regioselectively reacted aldimines to give an easy access to d-amino-b-keto esters18 with 1 equiv of an electrophile resulting in an enolate ion, and their diketone analogues. These compounds could easily which can be trapped by the addition of a second electrophile be converted into various piperidine and azetidine deriva- -

Tautomer Identification and Tautomer Structure Generation Based on The
J. Chem. Inf. Model. 2010, 50, 1223–1232 1223 Tautomer Identification and Tautomer Structure Generation Based on the InChI Code Torsten Thalheim,†,‡ Armin Vollmer,† Ralf-Uwe Ebert,† Ralph Ku¨hne,† and Gerrit Schu¨u¨rmann*,†,‡ UFZ Department of Ecological Chemistry, Helmholtz Centre for Environmental Research, Permoserstrasse 15, 04318 Leipzig, Germany, and Institute for Organic Chemistry, Technical University Bergakademie Freiberg, Leipziger Strasse 29, 09596 Freiberg, Germany Received March 27, 2010 An algorithm is introduced that enables a fast generation of all possible prototropic tautomers resulting from the mobile H atoms and associated heteroatoms as defined in the InChI code. The InChI-derived set of possible tautomers comprises (1,3)-shifts for open-chain molecules and (1,n)-shifts (with n being an odd number >3) for ring systems. In addition, our algorithm includes also, as extension to the InChI scope, those larger (1,n)-shifts that can be constructed from joining separate but conjugated InChI sequences of tautomer-active heteroatoms. The developed algorithm is described in detail, with all major steps illustrated through explicit examples. Application to ∼72 500 organic compounds taken from EINECS (European Inventory of Existing Commercial Chemical Substances) shows that around 11% of the substances occur in different heteroatom-prototropic tautomeric forms. Additional QSAR (quantitative structure-activity relationship) predictions of their soil sorption coefficient and water solubility reveal variations across tautomers up to more than two and 4 orders of magnitude, respectively. For a small subset of nine compounds, analysis of quantum chemically predicted tautomer energies supports the view that among all tautomers of a given compound, those restricted to H atom exchanges between heteroatoms usually include the thermodynamically most stable structures. -

Lecture 6 the Crossed Aldol Reaction and Its Many Variants
Lecture 6 The Crossed Aldol Reaction and its Many Variants Objectives: By the end of this lecture you will be able to: 1. make an appropriate choice of base to completely enolise carbonyl compounds; 2. use enolates in a crossed aldol reaction; 3. recognise the aldol functional group motif, and its variants, in complex molecules; 4. use the aldol disconnection to simplify a retrosynthetic analysis; 5. use the Reformatski and Mukaiyama aldol reactions in synthesis; 6. draw arrow-pushing mechanisms for all the aldol reactions discussed in this lecture. Introduction We have seen how choosing a base (B-) whose conjugate acid (B−H) is a much poorer acid − i.e. has a much higher pKa − than the proton we wish to abstract, ensures effectively complete deprotonation of the α-C−H of a carbonyl compound to provide the corresponding enolate. By completely enolising the carbonyl group, we can suppress self-condensation aldol processes, and instead use a different electrophile such as an aldehyde to form a β-hydroxy carbonyl compound. This reaction sequence is identical in basic mechanism to the intramolecular aldol processes that we discussed in previous lectures. It is now described as a crossed aldol condensation because the electrophile is a different carbonyl compound to the one that was used to form the nucleophilic enolate. This reaction, and its many variants, provides one of the most important methods for preparing C−C bonds. Pattern Recognition The aldol reaction and its many variants are very useful reactions in synthesis. You need to be able to identify the patterns or functional group motifs where this type of bond-forming process can be used.