Robert Burns Woodward
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Application of Organic Azides for the Synthesis of Nitrogen-Containing Molecules
ACCOUNT 21 Application of Organic Azides for the Synthesis of Nitrogen-Containing Molecules Shunsuke Chiba* Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore Fax +6567911961; E-mail: [email protected] Received 31 May 2012 Organic azides possess diverse chemical reactivities.4 Abstract: In this account, recent advances made on the reactions of several types of organic azides, such as vinyl azides, cyclic 2-azido Owing to their 1,3-dipole character, they undergo [3+2] alcohols, a-azido carbonyl compounds, towards the synthesis of cycloaddition with unsaturated bonds, such as those in nitrogen-containing molecules are described. alkynes and alkenes as well as carbonitriles (Scheme 1, part a).5 Organic azides can also be regarded as nitrene 1 Introduction equivalents (Scheme 1, part b).6 Accordingly, their reac- 2 Chemistry of Vinyl Azides tions with nucleophilic anions, electrophilic cations, and 2.1 Thermal [3+2]-Annulation of Vinyl Azides with 1,3-Dicar- radicals can formally provide the corresponding nitrogen bonyl Compounds 2.2 Manganese(III)-Catalyzed Formal [3+2]-Annulation with anions, cations, and radicals, respectively, forming a new 1,3-Dicarbonyl Compounds bond with the internal azido nitrogen and releasing molec- 2.3 Manganese(III)-Mediated/Catalyzed Formal [3+3]-Annu- ular nitrogen. Moreover, the generation of anions, cations, lation with Cyclopropanols and radicals at the a-position to the azido moiety can re- 2.4 Synthesis of Isoquinolines from a-Aryl-Substituted Vinyl sult in rapid denitrogenation to deliver the corresponding Azides and Internal Alkynes by Rhodium–Copper Bimetal- iminyl species, which can be used in further synthetic lic Cooperation transformations (i.e., carbon–nitrogen bond formation). -

Geoffrey Wilkinson
THE LONG SEARCH FOR STABLE TRANSITION METAL ALKYLS Nobel Lecture, December 11, 1973 by G EOFFREY W ILKINSON Imperial College of Science & Technology, London, England Chemical compounds in which there is a single bond between a saturated car- bon atom and a transition metal atom are of unusual importance. Quite aside from the significance and role in Nature of the cobalt to carbon bonds in the vitamin B 12 system and possible metal to carbon bonds in other biological systems, we need only consider that during the time taken to deliver this lec- ture, many thousands, if not tens of thousands of tons of chemical compounds are being transformed or synthesised industrially in processes which at some stage involve a transition metal to carbon bond. The nonchemist will pro- bably be most familiar with polyethylene or polypropylene in the form of do- mestic utensils, packaging materials, children’s toys and so on. These materials are made by Ziegler-Natta* or Philipps’ catalysis using titanium and chro- mium respectively. However, transition metal compounds are used as catalysts in the synthesis of synthetic rubbers and other polymers, and of a variety of simple compounds used as industrial solvents or intermediates. For example alcohols are made from olefins, carbon monoxide and hydrogen by use of cobalt or rhodium catalysts, acetic acid is made by carbonylation of methanol using rhodium catalysts and acrylonitrile is dimerised to adiponitrile (for nylon) by nickel catalysts. We should also not forget that the huge quantities of petroleum hydrocarbons processed by the oil and petrochemical industry are re-formed over platinum, platinum-rhenium or platinum-germanium sup- ported on alumina. -

Significance and Implications of Vitamin B-12 Reaction Shema- ETH ZURICH VARIANT: Mechanisms and Insights
Taylor University Pillars at Taylor University Student Scholarship: Chemistry Chemistry and Biochemistry Fall 2019 Significance and Implications of Vitamin B-12 Reaction Shema- ETH ZURICH VARIANT: Mechanisms and Insights David Joshua Ferguson Follow this and additional works at: https://pillars.taylor.edu/chemistry-student Part of the Analytical Chemistry Commons, Inorganic Chemistry Commons, Organic Chemistry Commons, Other Chemistry Commons, and the Physical Chemistry Commons CHEMISTRY THESIS SIGNIFICANCE AND IMPLICATIONS OF VITAMIN B-12 REACTION SCHEMA- ETH ZURICH VARIANT: MECHANISMS AND INSIGHTS DAVID JOSHUA FERGUSON 2019 2 Table of Contents: Chapter 1 6 Chapter 2 17 Chapter 3 40 Chapter 4 59 Chapter 5 82 Chapter 6 118 Chapter 7 122 Appendix References 3 Chapter 1 A. INTRODUCTION. Vitamin B-12 otherwise known as cyanocobalamin is a compound with synthetic elegance. Considering how it is composed of an aromatic macrocyclic corrin there are key features of this molecule that are observed either in its synthesis of in the biochemical reactions it plays a role in whether they be isomerization reactions or transfer reactions. In this paper the focus for the discussion will be on the history, chemical significance and total synthesis of vitamin B12. Even more so the paper will be concentrated one of the two variants of the vitamin B-12 synthesis, namely the ETH Zurich variant spearheaded by Albert Eschenmoser.Examining the structure as a whole it is observed that a large portion of the vitamin B12 is a corrin structure with a cobalt ion in the center of the macrocyclic part, and that same cobalt ion has cyanide ligands. -

Curriculum Vitae Markus Fischer Date of Birth 20 June 1962
Curriculum vitae Markus Fischer Date of birth 20 June 1962 Nationality German and Swiss Affiliation Institute of Plant Sciences, University of Bern Altenbergrain 21, 3013 Bern, Switzerland Phone +41 31 631 4943, Secretary 4911, Fax 4942 E-mail [email protected] Fields of Expertise Drivers and ecosystem-service consequences of biodiversity change Ecology and evolution of rare and invasive species Conservation biology, mountain ecology Work at the science-society and science-policy interfaces Employment Since 2007 Full Professor of Plant Ecology, Institute of Plant Sciences, University of Bern and (since 2010) Director of the Botanical Garden of the University of Bern Since 2012 Director, Institute of Plant Sciences, University of Bern Since 2012 Guest researcher, Senckenberg Biodiversity and Climate Institute, Frankfurt, Germany 2008-2011 Head, Biology Department, University of Bern 2007-2012 Guest Professor at the University of Potsdam, Germany 2003-2007 Full Professor of Botany and Community Ecology, Institute of Biochemistry and Biology, and Director of the Botanical Garden, University of Potsdam 1996-2003 Main Assistant, Institute of Environmental Sciences, Univ. of Zurich, Switzerland 1993-1996 Research Associate, Botanical Institute, University of Basel, Switzerland 1990 Research Associate, Department of Mechanical Engineering, University of Maryland, College Park, U.S.A. Education 1982-1989 Studies and MSc Diploma in Physics, Technical University Munich, Germany 1990–1992 Studies and BSc in Biology, University of Basel, -
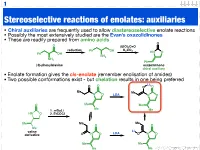
Stereoselective Reactions of Enolates: Auxiliaries
1 Stereoselective reactions of enolates: auxiliaries • Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions • Possibly the most extensively studied are the Evan’s oxazolidinones • These are readily prepared from amino acids O O (EtO)2C=O reduction K CO Ph OH 2 3 HN O Ph OH NH2 NH2 Ph (S)-phenylalanine oxazolidinone chiral auxiliary • Enolate formation gives the cis-enolate (remember enolisation of amides) • Two possible conformations exist - but chelation results in one being preferred Li O O O O Me Me N O LDA N O O Me Me Me 1. n-BuLi Me HN O 2. EtCOCl Me Me Me O O Me Li valine LDA derivative O N O O N O Me Me Me Me 123.702 Organic Chemistry 2 Diastereoselective alkylation of Evan’s enolate Li O O O O Me Me N O PhCH2I N O Ph Me Me Me Me I Ph Li O Bn O O O O O H H Me N Me N H Me Me Me Me iso-propyl group blocks bottom face • Clearly (I hope) one face of the enolate is blocked • Chelation results in a rigid structure that provides maximum steric hindrance • The electrophile can only approach from one face 123.702 Organic Chemistry 3 Diastereoselective alkylation of Evan’s enolate Li O O O O Me Me N O PhCH2I N O Ph Me Me Me Me I Ph Li O Bn O O O O O H H Me N Me N H Me Me Me Me iso-propyl group blocks bottom face • Clearly (I hope) one face of the enolate is blocked • Chelation results in a rigid structure that provides maximum steric hindrance • The electrophile can only approach from one face 123.702 Organic Chemistry 4 Diastereoselective functionalisation Li O O O O Br LDA Me Me N O N O H Ph Ph 96% de H O -

What Use Is Chemistry?
2 Inspirational chemistry What use is chemistry? Index 1.1 1 sheet This activity is based on a Sunday Times article by Sir Harry Kroto, a Nobel prize winning chemist who discovered a new allotrope of carbon – buckminsterfullerene or ‘bucky balls’. The article appeared on November 28, 2004 and is reproduced overleaf as a background for teachers. The aim is to introduce students to the scope of modern chemistry and the impact that it has on their lives, even in areas that they may not think of as related to chemistry. An alternative exercise for more able students would be to research what was used before chemical scientists had produced a particular new product or material (eg silk or wool stockings before nylon, leather footballs before synthetics, grated carbolic soap before shampoo) and then to write about the difference it would make to their lives if they did not have the modern product. Students will need: ■ Plenty of old magazines and catalogues (Argos catalogues are good as virtually everything in them would not exist without modern chemistry) ■ Large sheets of sugar paper ■ Glue and scissors. It works well if students produce the poster in groups, but then do the written work by themselves. The activity could be set for homework. Inspirational chemistry 3 What use is chemistry? Some years ago I was delighted chemistry-related industries make a to receive an honorary degree £5 billion profit on a £50 billion from Exeter University turnover, the apparent government recognising my contributions to inaction over the looming disaster chemistry – especially the is scarcely credible. -
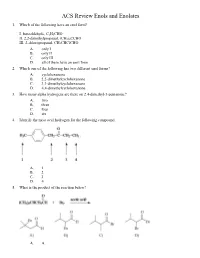
Ochem ACS Review 18 Enols and Enolates
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

Cambridge's 92 Nobel Prize Winners Part 2 - 1951 to 1974: from Crick and Watson to Dorothy Hodgkin
Cambridge's 92 Nobel Prize winners part 2 - 1951 to 1974: from Crick and Watson to Dorothy Hodgkin By Cambridge News | Posted: January 18, 2016 By Adam Care The News has been rounding up all of Cambridge's 92 Nobel Laureates, celebrating over 100 years of scientific and social innovation. ADVERTISING In this installment we move from 1951 to 1974, a period which saw a host of dramatic breakthroughs, in biology, atomic science, the discovery of pulsars and theories of global trade. It's also a period which saw The Eagle pub come to national prominence and the appearance of the first female name in Cambridge University's long Nobel history. The Gender Pay Gap Sale! Shop Online to get 13.9% off From 8 - 11 March, get 13.9% off 1,000s of items, it highlights the pay gap between men & women in the UK. Shop the Gender Pay Gap Sale – now. Promoted by Oxfam 1. 1951 Ernest Walton, Trinity College: Nobel Prize in Physics, for using accelerated particles to study atomic nuclei 2. 1951 John Cockcroft, St John's / Churchill Colleges: Nobel Prize in Physics, for using accelerated particles to study atomic nuclei Walton and Cockcroft shared the 1951 physics prize after they famously 'split the atom' in Cambridge 1932, ushering in the nuclear age with their particle accelerator, the Cockcroft-Walton generator. In later years Walton returned to his native Ireland, as a fellow of Trinity College Dublin, while in 1951 Cockcroft became the first master of Churchill College, where he died 16 years later. 3. 1952 Archer Martin, Peterhouse: Nobel Prize in Chemistry, for developing partition chromatography 4. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -

3.1.4 Droplet-Based Microfluidics
Rodriguez Garcia, Marc (2016) Engineering the transition from non-living to living matter. PhD thesis. https://theses.gla.ac.uk/7605/ Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten: Theses https://theses.gla.ac.uk/ [email protected] Engineering the transition from non-living to living matter Marc Rodríguez Garcia A thesis submitted to the University of Glasgow for the degree of Doctor of Philosophy School of Chemistry College of Science and Engineering August 2016 A tota la meva família, per haver-me ajudat a arribar fins aquí. Però sobretot als meus pares, per estar tant a prop malgrat la distància. I en especial a la Nuria, per ser el motiu que em fa tirar endavant. “The good thing about science is that it's true whether or not you believe in it.” -Neil deGrasse Tyson Acknowledgements 1 Acknowledgements This project was carried out between September 2012 and June 2016 in the group of Prof Leroy Cronin in the School of Chemistry at the University of Glasgow. I have received much help and support from many colleagues and friends. -

Chartered Status Charteredeverything You Need Tostatus Know Everything You Need to Know
Chartered Status CharteredEverything you need toStatus know Everything you need to know www.rsc.org/cchem www.rsc.org/cchem ‘The best of any profession is always chartered’ The RSC would like to thank its members (pictured top to bottom) Ben Greener, Pfizer, Elaine Baxter, Procter & Gamble, and Richard Sleeman, Mass Spec Analytical Ltd, for their participation and support . Chartered Status | 1 Contents About chartered status 3 Why become chartered? 3 What skills and experience do I need? 3 The professional attributes for a Chartered Chemist 5 Supporting you throughout the programme yThe Professional Development Programme 5 yThe Direct Programme 7 How to apply 7 Achieving Chartered Scientist status 8 Revalidation 8 The next step 8 Application form 9 2 | Chartered Status ‘Having a professionally recognised qualification will build my external credibility’ Elaine Baxter BSc PhD MRSC Procter & Gamble Elaine Baxter is a Senior Scientist at Procter & Gamble (P&G). Since joining the company, she has had roles in formulation, process and technology development in skin and shaving science. She graduated in 2001, before completing a PhD on synthetic inorganic chemistry of platinum dyes with applications in solar cells. Elaine is currently working towards Chartered Chemist status through the Professional Development Programme. Why do you want to achieve Chartered Chemist status? My role involves science communication with people such as dermatologists, academics and the media; having a professionally recognised qualification will build my external credibility with these professionals. How do you feel the programme has worked for you? Working towards achieving the attributes required for the CChem award has presented me with opportunities to share my industry knowledge and help others. -

Searching for Nucleic Acid Alternatives
MODIFIED OLIGONUCLEOTIDES 836 CHIMIA 2005, 59, No. 11 Chimia 59 (2005) 836–850 © Schweizerische Chemische Gesellschaft ISSN 0009–4293 Searching for Nucleic Acid Alternatives Albert Eschenmoser* Abstract: “Back of the envelope” methods have their place in experimental chemical research; they are effective mediators in the generation of research ideas, for instance, the design of molecular structures. Their qualitative character is part of their strength, rather than a drawback for the role they have to play. Qualitative conformational analysis of oligonucleotide and other oligomer systems on the level of idealized conformations is one such method; it has played a helpful role in our work on the chemical etiology of nucleic acid structure. This article, while giving a short overview of that work, shows how. Keywords: Conformational analysis of oligonucleotides · Nucleic acid analogs · Oligonucleodipeptides · p-RNA · TNA · Watson-Crick base-pairing Chemists understand by comparing, not ‘ab structural or transformational complexity, we know today as the molecular basis of initio’. To perceive and to create opportuni- serve the purpose of creating opportunities genetic function. The specific property to ties for drawing conclusions on the basis of to compare the behavior of complex sys- be compared in this work is a given nucleic comparisons is the organic chemist’s way tems with that of simpler ones. Enzymic re- acid alternative’s capacity for informational of interpreting and exploring the world at actions and enzyme models are examples.