GABAA Receptor Subtype- and Function-Selective Ligands: Key Issues in Translation to Humans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synaptic GABAA Receptor Clustering Without the 2 Subunit
The Journal of Neuroscience, July 30, 2014 • 34(31):10219–10233 • 10219 Cellular/Molecular ␥ Synaptic GABAA Receptor Clustering without the 2 Subunit Katalin Kerti-Szigeti, Zoltan Nusser, and X Mark D. Eyre Laboratory of Cellular Neurophysiology, Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest 1083, Hungary Rapid activation of postsynaptic GABAA receptors (GABAARs) is crucial in many neuronal functions, including the synchronization of neuronal ensembles and controlling the precise timing of action potentials. Although the ␥2 subunit is believed to be essential for the ␥ Ϫ/Ϫ postsynaptic clustering of GABAARs, synaptic currents have been detected in neurons obtained from 2 mice. To determine the role ␥ ␥ of the 2 subunit in synaptic GABAAR enrichment, we performed a spatially and temporally controlled 2 subunit deletion by injecting ␥ 77I Cre-expressing viral vectors into the neocortex of GABAAR 2 lox mice. Whole-cell recordings revealed the presence of miniature IPSCs in Cre ϩ layer 2/3 pyramidal cells (PCs) with unchanged amplitudes and rise times, but significantly prolonged decays. Such slowly decaying currents could be evoked in PCs by action potentials in presynaptic fast-spiking interneurons. Freeze-fracture replica immu- nogold labeling revealed the presence of the ␣1 and 3 subunits in perisomatic synapses of cells that lack the ␥2 subunit. Miniature IPSCs in Cre ϩ PCs were insensitive to low concentrations of flurazepam, providing a pharmacological confirmation of the lack of the ␥2 subunit. Receptors assembled from only ␣ subunits were unlikely because Zn 2ϩ did not block the synaptic currents. Pharmacological experiments indicated that the ␣␥3 receptor, rather than the ␣␦, ␣,or␣␥1 receptors, was responsible for the slowly decaying IPSCs.OurdatademonstratethepresenceofIPSCsandthesynapticenrichmentofthe␣1and3subunitsandsuggestthatthe␥3subunit ␥ is the most likely candidate for clustering GABAARs at synapses in the absence of the 2 subunit. -

BMC Pharmacology Biomed Central
CORE Metadata, citation and similar papers at core.ac.uk Provided by Springer - Publisher Connector BMC Pharmacology BioMed Central Research article Open Access Pharmacological Properties of DOV 315,090, an ocinaplon metabolite Dmytro Berezhnoy†1, Maria C Gravielle†1, Scott Downing1, Emmanuel Kostakis1, Anthony S Basile2, Phil Skolnick2, Terrell T Gibbs1 and David H Farb*1 Address: 1Laboratory of Molecular Neurobiology, Department of Pharmacology & Experimental Therapeutics, Boston University School of Medicine, 715 Albany St., Boston, MA 02118, USA and 2DOV Pharmaceutical, Inc, 150 Pierce St., Somerset, NJ 08873-4185, USA Email: Dmytro Berezhnoy - [email protected]; Maria C Gravielle - [email protected]; Scott Downing - [email protected]; Emmanuel Kostakis - [email protected]; Anthony S Basile - [email protected]; Phil Skolnick - [email protected]; Terrell T Gibbs - [email protected]; David H Farb* - [email protected] * Corresponding author †Equal contributors Published: 13 June 2008 Received: 20 December 2007 Accepted: 13 June 2008 BMC Pharmacology 2008, 8:11 doi:10.1186/1471-2210-8-11 This article is available from: http://www.biomedcentral.com/1471-2210/8/11 © 2008 Berezhnoy et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background: Compounds targeting the benzodiazepine binding site of the GABAA-R are widely prescribed for the treatment of anxiety disorders, epilepsy, and insomnia as well as for pre- anesthetic sedation and muscle relaxation. It has been hypothesized that these various pharmacological effects are mediated by different GABAA-R subtypes. -
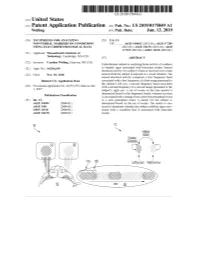
ANNNNNNNNNNNNNNNNNNNN 100A 006 Left Eye Input Right Eye Input
US 20190175049A1 ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2019 /0175049 A1 Welling ( 43 ) Pub . Date : Jun . 13 , 2019 ( 54 ) TECHNIQUES FOR ANALYZING (52 ) U . S . CI. NON -VERBAL MARKERS OF CONDITIONS CPC . .. A61B 5 /04842 (2013 . 01 ) ; A61B 5 / 7289 USING ELECTROPHYSIOLOGICAL DATA (2013 . 01) ; A61B 5 /0478 ( 2013 .01 ) ; A61B 5 /7225 ( 2013. 01 ) ; G06N 20 / 10 (2019 .01 ) (71 ) Applicant: Massachusetts Institute of Technology , Cambridge , MA (US ) ( 57 ) ABSTRACT (72 ) Inventor : Caroline Welling, Hanover, NH (US ) Embodiments related to analyzing brain activity of a subject to identify signs associated with binocular rivalry . Sensed ( 21 ) Appl. No. : 16 / 206, 639 electrical activity of a subject' s brain is received over a time period while the subject is exposed to a visual stimulus. The ( 22 ) Filed : Nov. 30 , 2018 sensed electrical activity comprises a first frequency band Related U . S . Application Data associated with a first frequency of a first image presented to the subject ' s left eye , a second frequency band associated (60 ) Provisional application No .62 / 593 , 535, filed on Dec . with a second frequency of a second image presented to the 1 , 2017 subject ' s right eye . A set of events in the time period is determined based on the frequency bands, wherein an event Publication Classification is associated with a change from a previous perceptual event (51 ) Int. Ci. to a new perceptual event. A metric for the subject is A61B 5 /0484 ( 2006 .01 ) determined based on the set of events . The metric is ana A61B 5 /00 ( 2006 .01 ) lyzed to determine whether the subject exhibits signs asso GO6N 20 / 10 (2006 .01 ) ciated with a condition that is associated with binocular A61B 5 /0478 ( 2006 .01 ) rivalry . -

18 December 2020 – to Date)
(18 December 2020 – to date) MEDICINES AND RELATED SUBSTANCES ACT 101 OF 1965 (Gazette No. 1171, Notice No. 1002 dated 7 July 1965. Commencement date: 1 April 1966 [Proc. No. 94, Gazette No. 1413] SCHEDULES Government Notice 935 in Government Gazette 31387 dated 5 September 2008. Commencement date: 5 September 2008. As amended by: Government Notice R1230 in Government Gazette 32838 dated 31 December 2009. Commencement date: 31 December 2009. Government Notice R227 in Government Gazette 35149 dated 15 March 2012. Commencement date: 15 March 2012. Government Notice R674 in Government Gazette 36827 dated 13 September 2013. Commencement date: 13 September 2013. Government Notice R690 in Government Gazette 36850 dated 20 September 2013. Commencement date: 20 September 2013. Government Notice R104 in Government Gazette 37318 dated 11 February 2014. Commencement date: 11 February 2014. Government Notice R352 in Government Gazette 37622 dated 8 May 2014. Commencement date: 8 May 2014. Government Notice R234 in Government Gazette 38586 dated 20 March 2015. Commencement date: 20 March 2015. Government Notice 254 in Government Gazette 39815 dated 15 March 2016. Commencement date: 15 March 2016. Government Notice 620 in Government Gazette 40041 dated 3 June 2016. Commencement date: 3 June 2016. Prepared by: Page 2 of 199 Government Notice 748 in Government Gazette 41009 dated 28 July 2017. Commencement date: 28 July 2017. Government Notice 1261 in Government Gazette 41256 dated 17 November 2017. Commencement date: 17 November 2017. Government Notice R1098 in Government Gazette 41971 dated 12 October 2018. Commencement date: 12 October 2018. Government Notice R1262 in Government Gazette 42052 dated 23 November 2018. -

Guaiana, G., Barbui, C., Caldwell, DM, Davies, SJC, Furukawa, TA
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Explore Bristol Research Guaiana, G., Barbui, C., Caldwell, D. M., Davies, S. J. C., Furukawa, T. A., Imai, H., ... Cipriani, A. (2017). Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta-analysis. Cochrane Database of Systematic Reviews, 2017(7), [CD012729]. https://doi.org/10.1002/14651858.CD012729 Publisher's PDF, also known as Version of record Link to published version (if available): 10.1002/14651858.CD012729 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Cochrane Library at https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012729/full . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/pure/about/ebr-terms Cochrane Database of Systematic Reviews Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta-analysis (Protocol) Guaiana G, Barbui C, Caldwell DM, Davies SJC, Furukawa TA, Imai H, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A Guaiana G, Barbui C, Caldwell DM, Davies SJC, Furukawa TA, Imai H, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A. Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta-analysis. Cochrane Database of Systematic Reviews 2017, Issue 7. -

Effect of Repeated Gaboxadol Administration on Night Sleep and Next-Day Performance in Healthy Elderly Subjects
Neuropsychopharmacology (2005) 30, 833–841 & 2005 Nature Publishing Group All rights reserved 0893-133X/05 $30.00 www.neuropsychopharmacology.org Effect of Repeated Gaboxadol Administration on Night Sleep and Next-Day Performance in Healthy Elderly Subjects 1 2 ,3 1 Stefan Mathias , Josef Zihl , Axel Steiger* and Marike Lancel 1Section of Sleep Pharmacology, Max-Planck-Institute of Psychiatry, Munich, Germany; 2Section of Neuropsychology, Max-Planck-Institute of Psychiatry, Munich, Germany; 3Department of Psychiatry, Max-Planck-Institute of Psychiatry, Munich, Germany Aging is associated with dramatic reductions in sleep continuity and sleep intensity. Since gaboxadol, a selective GABAA receptor agonist, has been demonstrated to improve sleep consolidation and promote deep sleep, it may be an effective hypnotic, particularly for elderly patients with insomnia. In the present study, we investigated the effects of subchronic gaboxadol administration on nocturnal sleep and its residual effects during the next days in elderly subjects. This was a randomized, double-blind, placebo-controlled, balanced crossover study in 10 healthy elderly subjects without sleep complaints. The subjects were administered either placebo or 15 mg gaboxadol hydrochloride at bedtime on three consecutive nights. Sleep was recorded during each night from 2300 to 0700 h and tests assessing attention (target detection, stroop test) and memory function (visual form recognition, immediate word recall, digit span) were applied at 0900, 1400, and 1700 h during the following days. Compared with placebo, gaboxadol significantly shortened subjective sleep onset latency and increased self-rated sleep intensity and quality. Polysomnographic recordings showed that it significantly decreased the number of awakenings, the amount of intermittent wakefulness, and stage 1, and increased slow wave sleep and stage 2. -

Withdrawing Benzodiazepines in Primary Care
PC\/ICU/ ADTiriC • CNS Drugs 2009,-23(1): 19-34 KtVltW MKIIWLC 1172-7047/I»/O(X)1«119/S4W5/C1 © 2009 Adis Dato Intocmation BV. All rights reserved. Withdrawing Benzodiazepines in Primary Care Malcolm Luder} Andre Tylee^ and ]ohn Donoghue^ 1 Institute of Psychiatry, King's College London, London, England 2 John Moores University, Liverpool, Scotland Contents Abstract ' 19 1. Benzodiazepine Usage 22 2. Interventions 23 2.1 Simple interventions 23 2.2 Piiarmacoiogicai interventions 25 2.3 Psychoiogical Interventions 26 2.4 Meta-Anaiysis ot Various interventions 27 3. Outcomes 28 4. Practicai Issues 29 5. Otiier Medications 30 5.1 Antidepressants 30 5.2 Symptomatic Treatments 30 6. Conciusions 31 Abstract The use of benzodiazepine anxiolytics and hypnotics continues to excite controversy. Views differ from expert to expert and from country to country as to the extent of the problem, or even whether long-term benzodiazepine use actually constitutes a problem. The adverse effects of these drugs have been extensively documented and their effectiveness is being increasingly questioned. Discontinua- tion is usually beneficial as it is followed by improved psychomotor and cognitive functioning, particularly in the elderly. The potential for dependence and addic- tion have also become more apparent. The licensing of SSRIs for anxiety disorders has widened the prescdbers' therapeutic choices (although this group of medications also have their own adverse effects). Melatonin agonists show promise in some forms of insomnia. Accordingly, it is now even more imperative that long-term benzodiazepine users be reviewed with respect to possible discon- tinuation. Strategies for discontinuation start with primary-care practitioners, who are still the main prescdbers. -

Drug Class Review Newer Drugs for Insomnia
Drug Class Review Newer Drugs for Insomnia Final Update 2 Report October 2008 The Agency for Healthcare Research and Quality has not yet seen or approved this report. The purpose of Drug Effectiveness Review Project reports is to make available information regarding the comparative clinical effectiveness and harms of different drugs. Reports are not usage guidelines, nor should they be read as an endorsement of or recommendation for any particular drug, use, or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Update 1: July 2006 Original: December 2005 Susan Carson, MPH Marian S. McDonagh, PharmD Sujata Thakurta, MPA:HA Po-Yin Yen, MS Drug Effectiveness Review Project Marian McDonagh, PharmD, Principal Investigator Oregon Evidence-based Practice Center Mark Helfand, MD, MPH, Director Copyright © 2008 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 2 Drug Effectiveness Review Project The medical literature relating to the topic is scanned periodically (see http://www.ohsu.edu/xd/research/centers-institutes/evidence-based-policy- center/derp/documents/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report based on the information contained in the scan. Some portions of the report may not be up to date. Prior versions of this report can be accessed at the DERP website. Insomnia Page 2 of 87 Final Report Update 2 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION ............................................................................................................. 6 Scope and Key Questions ...................................................................................................................... -

Pexion, Imepitoin
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Pexion 100 mg tablets for dogs Pexion 400 mg tablets for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains: Active substance: Imepitoin 100 mg Imepitoin 400 mg For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablets White, oblong, half-scored tablets with embedded logo “I 01” (100 mg) or “I 02” (400 mg) on one side. The tablet can be divided into equal halves. 4. CLINICAL PARTICULARS 4.1 Target species Dog 4.2 Indications for use, specifying the target species For the reduction of the frequency of generalised seizures due to idiopathic epilepsy in dogs for use after careful evaluation of alternative treatment options. 4.3 Contraindications Do not use in case of hypersensitivity to the active substance or to any of the excipients. Do not use in dogs with severely impaired hepatic function, severe renal or severe cardiovascular disorders (see section 4.7). 4.4 Special warnings The pharmacological response to imepitoin may vary and efficacy may not be complete. Nevertheless imepitoin is considered to be a suitable treatment option in some dogs because of its safety profile (see section 5.1). On treatment, some dogs will be free of seizures, in other dogs a reduction of the number of seizures will be observed, whilst others may be non-responders. In non-responders, an increase in seizure frequency may be observed. Should seizures not be adequately controlled, further diagnostic measures and other antiepileptic treatment should be considered. The benefit/risk assessment for the individual dog should take into account the details in the product literature. -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Dynamic Regulation of the GABAA Receptor Function by Redox Mechanisms S
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2016/07/20/mol.116.105205.DC1 1521-0111/90/3/326–333$25.00 http://dx.doi.org/10.1124/mol.116.105205 MOLECULAR PHARMACOLOGY Mol Pharmacol 90:326–333, September 2016 Copyright ª 2016 by The American Society for Pharmacology and Experimental Therapeutics MINIREVIEW—A LATIN AMERICAN PERSPECTIVE ON ION CHANNELS Dynamic Regulation of the GABAA Receptor Function by Redox Mechanisms s Daniel J. Calvo and Andrea N. Beltrán González Laboratorio de Neurobiología Celular y Molecular, Instituto de Investigaciones en Ingeniería Genética y Biología Molecular Downloaded from ¨Dr. Héctor N. Torres¨ (INGEBI), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ciudad Autónoma de Buenos Aires, Argentina (D.J.C., A.N.B.G.) Received May 15, 2016; accepted July 14, 2016 ABSTRACT molpharm.aspetjournals.org Oxidizing and reducing agents, which are currently involved normally present in neurons and glia or are endogenously in cell metabolism and signaling pathways, can regulate fast generated in these cells under physiologic states or during inhibitory neurotransmission mediated by GABA receptors in the oxidative stress (e.g., hydrogen peroxide, superoxide and hy- nervous system. A number of in vitro studies have shown that droxyl radicals, nitric oxide, ascorbic acid, and glutathione), diverse redox compounds, including redox metabolites and induce potentiating or inhibiting actions on different native and reactive oxygen and nitrogen species, modulate phasic and recombinant GABAA receptor subtypes. Based on these results, it tonic responses mediated by neuronal GABAA receptors through is thought that redox signaling might represent a homeostatic both presynaptic and postsynaptic mechanisms. -

Neurobiological and Neurobehavioral Mechanisms of Chronic Alcohol Drinking
Neurobiological and Neurobehavioral Mechanisms of Chronic Alcohol Drinking Neurobiological and Neurobehavioral Mechanisms of Chronic Alcohol Drinking Alcoholism comprises a set of complex behaviors seeking behaviors, have been developed. These in which an individual becomes increasingly models, which remain an integral part of the preoccupied with obtaining alcohol. These study of alcoholism, include animals that pref- behaviors ultimately lead to a loss of control over erentially drink solutions containing alcohol, consumption of the drug and to the development animals that self-administer alcohol during of tolerance, dependence, and impaired social and withdrawal, animals with a history of dependence occupational functioning. that self-administer alcohol, and animals that self- administer alcohol after a period of abstinence Although valuable information regarding toler- from the drug. Genetic models for alcoholism ance and dependence has been, and continues to also exist and include animals that have been bred be, gathered through human studies, much of the selectively for high alcohol consumption. Studies detailed understanding of the impact of exposure using such models are uncovering the systemic, to alcohol on behavior and on the biological cellular, and molecular neurobiological mech- mechanisms underlying those behaviors has been anisms that appear to contribute to chronic obtained through the use of animal models for alcohol consumption. The challenge of current alcoholism and a variety of in vitro, or cellular, and future studies is to understand which specific systems. Through the use of cellular systems and cellular and subcellular systems undergo mole- animal models, researchers can control the genetic cular changes to influence tolerance and depen- background and experimental conditions under dence in motivational systems that lead to which a specific alcohol-related behavior or chronic drinking.