Anatomic Site Evaluation of the Zygomatic Bone for Dental Implant Placement Sorption Following Extraction, Trauma, In- to Increase the Bone Volume
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Results Description of the SKULLS. the Overall Size of Both Skulls Was Considered to Be Within Normal Limits for Their Ethnic
Ossification Defects and Craniofacial Morphology In Incomplete Forms of Mandibulofacial Dysostosis A Description of Two Dry Skulls ERIK DAHL, D.D.S., DR. ODONT. ARNE BJORK, D.D.S., ODONT. DR. Copenhagen, Denmark The morphology of two East Indian dry skulls exhibiting anomalies which were suggested to represent incomplete forms of mandibulofacial dysostosis is described. Obvious although minor ossification anomalies were found localized to the temporal, sphenoid, the zygomatic, the maxillary and the mandibular bones. The observations substantiate the concept of the regional and bilateral nature of this malformation syndrome. Bilateral orbital deviations, hypoplasia of the malar bones, and incomplete zygomatic arches appear to be hard tissue aberrations which may be helpful in exami- nation for subclinical carrier status. Changes in mandibular morphology seem to be less distinguishing features in incomplete or abortive types of mandibulofacial dysostosis. KEY WORDS craniofacial problems, mandible, mandibulofacial dysostosis, maxilla, sphenoid bone, temporal bone, zygomatic bone Mandibulofacial dysostosis (MFD) often roentgencephalometric examinations were results in the development of a characteristic made of the skulls, and tomograms were ob- facial disfigurement with considerable simi- tained of the internal and middle ear. Com- larity between affected individuals. However, parisons were made with normal adult skulls the symptoms may vary highly in respect to and with an adult skull exhibiting the char- type and degree, and both incomplete and acteristics of MFD. All of the skulls were from abortive forms of the syndrome have been the same ethnic group. ' reported in the literature (Franceschetti and Klein, 1949; Moss et al., 1964; Rogers, 1964). Results In previous papers, we have shown the DEsCRIPTION OF THE SKULLS. -

Three-Dimensional Radiographic Evaluation of the Malar Bone Engagement Available for Ideal Zygomatic Implant Placement
Article Three-Dimensional Radiographic Evaluation of the Malar Bone Engagement Available for Ideal Zygomatic Implant Placement Gerardo Pellegrino 1,* , Francesco Grande 2 , Agnese Ferri 1, Paolo Pisi 3, Maria Giovanna Gandolfi 4 and Claudio Marchetti 1 1 Oral and Maxillofacial Surgery Unit, Department of Biomedical and Neuromotor Sciences, University of Bologna, 40125 Bologna, Italy; [email protected] (A.F.); [email protected] (C.M.) 2 Oral Surgery Unit, Dental School, Department of Biomedical and Neuromotor Sciences, University of Bologna, 40125 Bologna, Italy; [email protected] 3 Dental Radiology Unit, Dental School, Department of Biomedical and Neuromotor Sciences, University of Bologna, 40125 Bologna, Italy; [email protected] 4 Medical-technical Science, Dental School, Department of Biomedical and Neuromotor Sciences, University of Bologna, 40125 Bologna, Italy; mgiovanna.gandolfi@unibo.it * Correspondence: [email protected]; Tel.: +39-051-208-8157 Received: 11 June 2020; Accepted: 21 July 2020; Published: 22 July 2020 Abstract: Zygomatic implant rehabilitation is a challenging procedure that requires an accurate prosthetic and implant plan. The aim of this study was to evaluate the malar bone available for three-dimensional zygomatic implant placement on the possible trajectories exhibiting optimal occlusal emergence. After a preliminary analysis on 30 computed tomography (CT) scans of dentate patients to identify the ideal implant emergencies, we used 80 CT scans of edentulous patients to create two sagittal planes representing the possible trajectories of the anterior and posterior zygomatic implants. These planes were rotated clockwise on the ideal emergence points and three different hypothetical implant trajectories per zygoma were drawn for each slice. -

Zygomatic Fractures
Without treatment in a timely Zygomatic Fractures manner, many individuals will develop future problems, the severity and consequences of which can be much greater than if the injury had been immediately repaired. However, modern cran- iofacial surgical techniques can now offer hope for patients with pre-existing post-traumatic facial deformities despite considerable delays between injury, diagnosis, Front and lateral three dimensional CT Scans demonstrate and treatment. These innovative displaced fractures of all zygomatic buttresses. techniques establish a higher stan- dard of care for the management of facial injuries. The following sections describe the different areas and types of facial fractures: ZYGOMATIC FRACTURES The zygomatic bone occupies a prominent and important posi- tion in the facial skeleton. It plays a key role in determining facial width as well as acting as a major Patient with a left displaced An open reduction with rigid buttress of the midface. Its anteri- zygomatic fracture. miniplate fixation was performed or projection forms the malar emi- with postoperative result shown. nence and is often referred to as the malar bone. The zygoma has several important articulations in the midface. The zygoma forms a significant portion of the floor and object, or secondary to motor vehicle accidents. Moderate lateral wall of the orbit. In addi- force may result in minimally or nondisplaced fractures at tion, the zygoma meets the lateral the suture lines. More severe blows frequently result in skull to form the zygomatic arch. inferior, medial, and posterior displacement of the zygoma. Comminuted fractures of the body with separation at the The zygoma is the main but- suture lines are most often the result of high-velocity motor tress between the maxilla and the vehicle accidents. -

Comparative Anatomy of the Human and Horse Orbit
Revista Română de Anatomie funcţională şi clinică, macro- şi microscopică şi de Antropologie Vol. XIX – Nr. 1 – 2020 ORIGINAL PAPERS COMparaTIVE AnaTOMY OF THE HUMAN AND Horse ORBIT Ruxandra Coroleucă1, F.M. Filipoiu2, O. Munteanu2*, M. Enyedi2 University of Medicine and Pharmacy „Carol Davila” Bucharest 1. „Carol Davila” – Doctoral School 2. Department of Anatomy COmpaRATIVE ANATOMY OF THE HUMAN AND HORSE ORBIT (Abstract): Starting from the definition of evolution itself, it describes a process through which a living organism has grown and developed from earlier forms over successive generations. The phylogenetics shows us differ- ent evolutionary development and diversification for different species and this comparative anato- my study aims to highlight the similarities and the differences between human and horse bony orbit. We conducted a study of comparative anatomy of human and horse orbit in which we first observed the two orbits and then identified data about the location, the constitutive walls of the orbits and the differences and similarities between them. The evolution from the four-legged posi- tion to the bipedal position was decisive for the location and the orbital characteristics. Key-words: HUMAN ORBIT, HORSE ORBIT, COMPARATIVE ANATOMY INTRODUCTION modern horses the orbit is located superior and In osteology, the skull is perhaps the most slightly posterior to the terminal portion of the studied anatomical segment of the skeleton, it row of teeth, in contrast to the primitive posi- is also the basis for establishing a biological tion above the first molar. The direction of the and personal identity. The skeleton of the skull orbit is lateral (2). -

Zygomatic Bone: Anatomic Bases for Osseointegrated Implant Anchorage
Rigolizzo 5/19/05 4:07 PM Page 441 Zygomatic Bone: Anatomic Bases for Osseointegrated Implant Anchorage Maurício Bruhns Rigolizzo, DDS, MS1/José Angelo Camilli, MS, PhD2/ Carlos Eduardo Francischone, DDS, MS, PhD3/Carlos Roberto Padovani, MS, PhD4/Per-Ingvar Brånemark, MD, PhD5 Purpose: The aim of the present study was to evaluate zygomatic bone thickness considering a possi- ble relationship between this parameter and cephalic index (CI) for better use of CI in the implant placement technique. Materials and Methods: CI was calculated for 60 dry Brazilian skulls. The zygo- matic bones of the skulls were divided into 13 standardized sections for measurement. Bilateral mea- surements of zygomatic bone thickness were made on dry skulls. Results: Sections 5, 6, 8, and 9 were appropriate for implant anchorage in terms of location. The mean thicknesses of these sections were 6.05 mm for section 5, 3.15 mm for section 6, 6.13 mm for section 8, and 4.75 mm for section 9. In only 1 section, section 8, did mean thickness on 1 side of of the skull differ significantly from mean thickness on the other side (P < .001). Discussion: For the relationship between quadrant thick- ness and CI, sections 6 and 8 varied independently of CI. Section 5 associated with brachycephaly, and section 9 associated with subbrachycephaly, presented variations in the corresponding thickness. Conclusion: Based on the results, implants should be placed in sections 5 and 8, since they presented the greatest thickness, except in brachycephalic subjects, where thickness was greatest in section 5, and in subbrachycephalic subjects, where thickness was greatest in section 9. -

Axis Scientific 22-Part Osteopathic Natural Bone Human Skull A-105940
Axis Scientific 22-Part Osteopathic Natural Bone Human Skull A-105940 Frontal Bone Nasal Bone Nasal Bone (Right) (Left) Frontal Bone Ethmoid Bone Parietal Bone Parietal Bone (Right) (Left) Parietal Bone Lacrimal (Right) Bone (Right) Temporal Bone Nasal Bone Temporal Bone (Left) (Right) (Right) Sphenoid Bone Lacrimal Bone (Right) Lacrimal Bone (Left) R L Zygomatic Bone Zygomatic Bone (Right) (Left) Maxilla & Teeth Maxilla & Teeth (Right Side) (Left Side) Inferior Nasal Sphenoid Bone Inferior Nasal Concha (Left) Concha (Right) Occipital Bone Temporal Bone Zygomatic Bone (Right) (Right) Maxilla & Teeth Mandible & Mandible & (Right Side) Vomer Teeth Teeth Anterior View Lateral View Parietal Bone Parietal Bone (Right) (Left) Maxilla & Teeth Maxilla & Teeth (Right Side) (Left Side) Palatine Palatine Bone (Right) Bone (Left) Zygomatic Zygomatic Bone (Left) Bone (Right) Sphenoid Bone R L L R Vomer Temporal Bone Temporal Bone Temporal Bone (Right) (Left) (Right) Temporal Bone (Left) Parietal Bone Parietal Bone (Left) (Right) Occipital Bone Occipital Bone Mandible & Teeth Inferior View Posterior View Parietal Bone Frontal Bone Parietal Bone (Right) (Left) Parietal Bone Parietal Bone (Right) (Left) Temporal Temporal Bone (Right) Bone (Left) Temporal Bone Temporal Bone (Right) (Left) A Frontal Bone R L C B Sphenoid Sphenoid Sphenoid Bone Bone Bone Occipital Bone D E F Zygomatic Bone (Right) Zygomatic G Bone (Left) Lacrimal Bone (Right) Mandible & Maxilla & Teeth Maxilla & Teeth Maxilla & Teeth Teeth (Right Side) (Left Side) Maxilla & Teeth (Left Side) -

The Relationship Between Skull Morphology, Masticatory Muscle Force
Annals of Anatomy 203 (2016) 59–68 Contents lists available at ScienceDirect Annals of Anatomy jou rnal homepage: www.elsevier.com/locate/aanat The relationship between skull morphology, masticatory muscle force ଝ and cranial skeletal deformation during biting a,b,c,∗ d a Viviana Toro-Ibacache , Víctor Zapata Munoz˜ , Paul O’Higgins a Department of Archaeology and Hull York Medical School, University of York, Heslington, York YO10 5DD, United Kingdom b Facultad de Odontología, Universidad de Chile, Sergio Livingstone Pohlhammer 943, Independencia, Región Metropolitana, Chile c Max Planck Institute for Evolutionary Anthropology, Department of Human Evolution, Deutscher Platz 6, 04103 Leipzig, Germany d Centro de Imagenología, Hospital Clínico Universidad de Chile, Santos Dumont 999, Independencia, Región Metropolitana, Chile a r a t i c l e i n f o b s t r a c t Article history: The human skull is gracile when compared to many Middle Pleistocene hominins. It has been argued Received 28 November 2014 that it is less able to generate and withstand high masticatory forces, and that the morphology of the Received in revised form 27 February 2015 lower portion of the modern human face correlates most strongly with dietary characteristics. This study Accepted 1 March 2015 uses geometric morphometrics and finite element analysis (FEA) to assess the relationship between skull morphology, muscle force and cranial deformations arising from biting, which is relevant in under- Keywords: standing how skull morphology relates to mastication. The three-dimensional skull anatomies of 20 Modern humans individuals were reconstructed from medical computed tomograms. Maximal contractile muscle forces Skull morphology were estimated from muscular anatomical cross-sectional areas (CSAs). -

Zygomatic Bone Shape in Intentional Cranial
Zygomatic bone shape in intentional cranial deformations: a model for the study of the interactions between skull growth and facial morphology Serge Ketoff, François Girinon, Stefan Schlager, Martin Friess, Thomas Schouman, Philippe Rouch, Roman Hossein Khonsari To cite this version: Serge Ketoff, François Girinon, Stefan Schlager, Martin Friess, Thomas Schouman, et al.. Zygo- matic bone shape in intentional cranial deformations: a model for the study of the interactions be- tween skull growth and facial morphology. Journal of Anatomy, Wiley, 2016, 230 (4), pp.524-531. 10.1111/joa.12581. hal-02486640 HAL Id: hal-02486640 https://hal.archives-ouvertes.fr/hal-02486640 Submitted on 21 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Zygomatic bone shape in intentional cranial deformations: a model for the study of the interactions between skull growth and facial morphology S. Ketoff,1,2,3 F. Girinon,3 S. Schlager,4 M. Friess,5 T. Schouman,1,2,3 P. Rouch3 and R. H. Khonsari1,2 1Assistance Publique - Hopitaux^ de Paris, Hopital^ Universitaire -
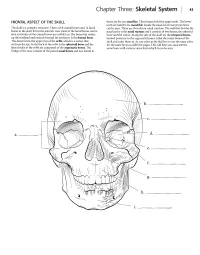
Skeletal System 43
Chapter Three: Skeletal System 43 FRONTAL ASPECT OF THE SKULL them are the two maxillae. These bones hold the upper teeth. The lower teeth are held by the mandible. Inside the nasal cavity two projections The skull is a complex structure. There are 8 cranial bones and 14 facial can be seen. These are the inferior nasal conchae. The wall that divides the bones in the skull. From the anterior view most ofthe facial bones can be nasal cavity is the nasal septum and it consists of two bones, the ethmoid seen and some of the cranial bones are visible too. The bone that makes bone and the vomer. Along the side of the skull are the temporal bones, up the forehead and extends beyond the eyebrows is the frontal bone. located posterior to the zygomatic bones. Label the major bones of the This bone forms the upper rim of the orbit, which is a socket that skull and color them in. As you color in the skull try to use the same color encloses the eye.In the back of the orbit is the sphenoid bone and the for the same bone on different pages. This will help you associate the lateral walls of the orbit are composed of the zygomatic bones. The same bone with various views from which it can be seen. bridge of the nose consists of the paired nasal bones and just lateral to a. _ e. --------_ f. g.------- h. Answer Key:a. Orbit,b. Frontal bone, c.Temporal bone, d. Sphenoid bone, e. -

Anatomical and Radiographic Study of the White-Eared Opossum (Didelphis Albiventris) Skull1
Pesq. Vet. Bras. 36(11):1132-1138, novembro 2016 DOI: 10.1590/S0100-736X2016001100013 Anatomical and radiographic study of the white-eared opossum (Didelphis albiventris) skull1 2,4 3 3 2 4 4,5 Bruno C. Schimming *, Luís Felipe F. Reiter , Lívia M. Sandoval , André L. ABSTRACT.-Filadelpho , Letícia R. Inamassu and Maria Jaqueline Mamprim Anatomical and radiographic study of the white-eared opossum (Didelphis albiventris Schimming) skull.B.C., Reiter Pesquisa L.F.F., Veterinária Sandoval BrasileiraL.M., Filadelpho 36(11):1132-1138 A.L., Inamassu L.R. &- Mamprim M.J. 2016. Departa mento de Anatomia, Universidade Estadual Paulista, Cx. Postal 510, Distrito de Rubião Jr s/n, Botucatu, SP 18618-970, Brazil. E-mail: [email protected] This study was made to investigate the anatomical features of the white-eared opossum skull, by osteology and radiographic anatomy. For this, five animals were used without sexual distinction. The skull was examined by radiographic and macroscopic characteristics. The skulls were then subjected to maceration. The skull was described macroscopically according to standard views, i.e. dorsal and caudal, lateral, ventral, and midsagittal. The skull can be- divided into facial (viscerocranium) and cranial (neurocranium) regions. The facial region was elongated and more developed than neurocranium. The supraorbital foramen was ab sent. The tympanic bulla is not well developed. The zygomatic arch was formed by zygomatic process of the temporal bone, zygomatic process of the maxilla, and temporal process of the zygomatic bone. There was no significant difference between bones found in this study when compared with those described for others mammals. -

Surgical Orbital Anatomy
85 Surgical Orbital Anatomy Shirley Hu, MD1,2 Patrick Colley, MD1,2 1 Department of Otolaryngology, Mount Sinai Medical Center, New Address for correspondence Shirley Hu, MD, 310 East 14th Street, York, New York New York, New York 10003 (e-mail: [email protected]). 2 Department of Otolaryngology, New York Eye and Ear Infirmary of MountSinai,NewYork,NewYork Semin Plast Surg 2019;33:85–91. Abstract In this article, the anatomy of the orbit is reviewed, with aspecific emphasis on surgical anatomy. A brief discussion of the ocular globe is also included. The orbits are pyramidal structures separating the upper and middle facial skeletons. The walls, Keywords apex, and base harbor several foramina and fissures as well as bony irregularities where ► orbital anatomy various ligaments, muscles, and capsules attach. There are a variety of surgical ► surgical approaches to the orbit, including the traditional transcutaneous and neurosurgical ► globe techniques and, more recently, minimally invasive, endoscopic approaches. The orbit is a pyramidal structure that encompasses the beyond the inferior orbital fissure, and then gently curves up organ of vision and separates the upper and middle facial toward the superior orbital fissure. When repairing orbital skeletons, with its apex located posteriorly and base situated floor fractures, recreating this subtle curvature will restore anteriorly. The bone comprising the apex and base is much normal anatomyand help prevent malpositioning of the globe.7 thicker than that of the walls, allowing the apex to protect the brain and optic nerve from direct force and the orbital Medial Orbital Wall rim to resist fracture. Pressure to the globe is thus dispersed The medial orbital wall is in the sagittal plane and has the to the curvilinear orbital walls, which serve to maintain the greatest degree of cephalocaudad curvature. -

Fractures of the Zygomatic Complex
FRACTURES OF THE ZYGOMATIC COMPLEX RUSSELL HOPKINS M.R.C.S., L.R.C.P., B.D.S., F.D.S.R.C.S. Consultant in Oral Surgery, University Hospital of Wales, Cardiff Anatomy THE FRONTAL PROCESS of the zygomatic bone articulates superiorly with the frontal bone at the zygomatico-frontal suture, giving attachment on its orbital aspect to the suspensory ligament of the eye (Lockwood's) at the lateral orbital tubercle (Whitnall's). Posteriorly the zygomatic process of the zygomatic bone articulates with the zygomatic process of SUBCONJUNCTIVAL HAEMORRHAGE Fig. 1. A diagrammatic representation of the anatomical basis of the common signs and symptoms of fracture of the zygomatic complex. Minor occlusal disturbance occurs if there is an associated alveolar fracture. the temporal bone to form the zygomatic arch. The temporal fascia is inserted into the upper aspect of the arch and deep to it lies the temporalis muscle attached to the coronoid process of the mandible. Antero-inferiorly the bone articulates with the zygomatic process of the maxilla forming the lateral two-thirds of the inferior orbital margin and enclosing the infra-orbital nerve. In the orbit articulating with the greater wing of sphenoid above and the maxilla below, the bone forms part of the lateral boundary of the inferior orbital fissure. The zygo- matic bone forms the superior antero-lateral boundary of the maxillary antrum and, being part of the orbital skeleton, affords considerable protection to the eye. Postgraduate Lecture (Ann. Roy. Coll. Surg. Engl. 1971, vol. 49) 403 RUSSELL HOPKINS The area is referred to in this paper as the 'zygomatic complex' but the 'malar' or 'lateral middle third' is also used.