Download This Article in PDF Format
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ACC JOURNAL 2020, Volume 26, Issue 2 DOI: 10.15240/Tul/004/2020-2-002
ACC JOURNAL 2020, Volume 26, Issue 2 DOI: 10.15240/tul/004/2020-2-002 THE DEVELOPMENT OF THE NONPROFIT SECTOR IN RUSSIAN REGIONS: MAIN CHALLENGES Anna Artamonova Vologda Research Center of the Russian Academy of Sciences, Department of Editorial-and-Publishing Activity and Science-Information Support, 56A, Gorky str., 160014, Vologda, Russia e-mail: [email protected] Abstract This article aims at identifying the main barriers hindering development of the nonprofit sector in Russian regions. The research is based on the conviction that the development of the nonprofit sector is crucial for the regional socio-economic system and depends upon civic engagement. The results of an analysis of available statistical data and a sociological survey conducted in one of the Russian regions reveal that the share of the Russians engaged in volunteer activities is low; over 80% of the population do not participate in public activities; less than 10% have definite knowledge of working nonprofit organizations. The study allowed identifying three groups of the main barriers and formulating some recommendations for their overcoming. Keywords Russia; Nonprofit sector; Nongovernmental organization; Civic participation; Civic engagement. Introduction Sustainable development of Russian regions requires the fullest use of their internal potential. As the public and private sectors cannot meet all demands concerning the provision of high living standards for all groups of the population, it is necessary for local authorities to find new opportunities for effective and mutually beneficial cooperation with other economic actors. In Russian regions, in this regard a new trend becomes evident government starts to pay more attention to organizations of the third (nonprofit) sector. -

Peter the Great and His Changing Identity Emily Frances Pagrabs Wofford College
Wofford College Digital Commons @ Wofford Student Scholarship 5-2016 Peter the Great and His Changing Identity Emily Frances Pagrabs Wofford College Follow this and additional works at: http://digitalcommons.wofford.edu/studentpubs Part of the European History Commons, and the Slavic Languages and Societies Commons Recommended Citation Pagrabs, Emily Frances, "Peter the Great and His Changing Identity" (2016). Student Scholarship. Paper 17. http://digitalcommons.wofford.edu/studentpubs/17 This Honors Thesis is brought to you for free and open access by Digital Commons @ Wofford. It has been accepted for inclusion in Student Scholarship by an authorized administrator of Digital Commons @ Wofford. For more information, please contact [email protected]. Peter the Great and His Changing Identity Senior History Honors Thesis May 11, 2016 Emiley Pagrabs Pagrabs 1 Introduction Well aware of the perception that foreigners held of him, Peter the Great would never apologize for his nationality or his country. A product of his upbringing, Peter did have some qualities that many foreigners criticized as barbaric and harsh. Said Peter: They say that I am cruel; that is what foreigners think of me, but who are they to judge? They do not know what the situation was at the beginning of my reign, and how many were opposed to my plans, and brought about the failure of projects which would have been of great benefit to my country obliging me to arm myself with great severity; but I have never been cruel…I have always asked for the cooperation of those of my subjects in whom I have perceived intelligence and patriotism, and who, agreeing with my views, were ready to support them.1 Essentially, Peter I was simply a Russian. -

St Petersburg 8
Plan Your Trip 12 ©Lonely Planet Publications Pty Ltd St Petersburg “All you’ve got to do is decide to go and the hardest part is over. So go!” TONY WHEELER, COFOUNDER – LONELY PLANET Regis St Louis, Simon Richmond Contents PlanPlan Your Your Trip Trip page 1 4 Welcome to Top Itineraries ���������������20 Travelling to Moscow ����36 St Petersburg ������������������ 4 If You Like� ����������������������22 Museums St Petersburg’s Month by Month ������������24 & Galleries �������������������37 Top 10 ������������������������������� 6 With Kids ������������������������26 Eating ���������������������������39 What’s New �������������������� 13 Money-Saving Tips �������28 Drinking Need to Know �����������������14 & Nightlife ������������������ 43 Visas �������������������������������29 First Time Entertainment ������������ 46 St Petersburg �����������������16 Tours & Activities �����������31 Shopping ��������������������� 48 Getting Around �������������� 18 Visiting on a Cruise �������34 Explore St Petersburg 50 Historic Heart ����������������54 Vasilyevsky Island ������� 143 Day Trips from Sennaya & Kolomna ���104 Petrograd & St Petersburg ������������ 173 Vyborg Sides ��������������� 154 Smolny & Sleeping ���������������������186 Vosstaniya ��������������������121 Understand St Petersburg 197 St Petersburg History ������������������������� 200 Arts �������������������������������226 Today ���������������������������� 198 Architecture ����������������� 219 Literature ���������������������236 Survival Guide 241 Transport ���������������������242 -

The Siege of Leningrad (1941-1944)
War fronts The siege of Leningrad (1941-1944) François-Xavier NÉRARD ABSTRACT Lasting 900 days between September 1941 and January 1944, the siege of Leningrad claimed the lives of 800,000 of the city’s inhabitants, mainly through cold and hunger. The population of the city was subjected, moreover, to enemy fire and to ruthlessly strict control by the Soviet authorities. The memory of the suffering of Leningrad’s population was first celebrated, then stifled, and is only gradually re-emerging. Tanya Savicheva's Diary The siege of Leningrad by German and Finnish forces (as well as the soldiers of the Division Azul, Spanish volunteers) is a key episode in the Second World War on Soviet territory and saw the reappearance of a form of warfare that was thought to have died out in the nineteenth century. Although less present in narratives of the war in the West, the siege was a major traumatic event for the USSR and Russia. As a symbol of resistance and suffering, it differs from Stalingrad, a heroic victory that could be celebrated more easily. Of Leningrad’s 2.5 million inhabitants on the eve of the conflict, only 600,000 were still alive in the city when it was liberated by the Red Army on 27 January 1944, around one million having been evacuated before and during the siege. It is estimated today that 800,000 people died in the siege, mostly from cold and hunger. Leningrad, along with Moscow and Kiev, was one of the major objectives of the German offensive launched on 21 June 1941, but the city was not taken during the attack. -

Science of Economics
ACC JOURNAL XXVI 2/2020 Issue B Science of Economics TECHNICKÁ UNIVERZITA V LIBERCI HOCHSCHULE ZITTAU/GÖRLITZ INTERNATIONALES HOCHSCHULINSTITUT ZITTAU (TU DRESDEN) UNIWERSYTET EKONOMICZNY WE WROCŁAWIU WYDZIAŁ EKONOMII, ZARZĄDZANIA I TURYSTYKI W JELENIEJ GÓRZE Indexed in: Liberec – Zittau/Görlitz – Wrocław/Jelenia Góra © Technická univerzita v Liberci 2020 ISSN 1803-9782 (Print) ISSN 2571-0613 (Online) ACC JOURNAL je mezinárodní vědecký časopis, jehož vydavatelem je Technická univerzita v Liberci. Na jeho tvorbě se podílí čtyři vysoké školy sdružené v Akademickém koordinačním středisku v Euroregionu Nisa (ACC). Ročně vycházejí zpravidla tři čísla. ACC JOURNAL je periodikum publikující původní recenzované vědecké práce, vědecké studie, příspěvky ke konferencím a výzkumným projektům. První číslo obsahuje příspěvky zaměřené na oblast přírodních věd a techniky, druhé číslo je zaměřeno na oblast ekonomie, třetí číslo pojednává o tématech ze společenských věd. ACC JOURNAL má charakter recenzovaného časopisu. Jeho vydání navazuje na sborník „Vědecká pojednání“, který vycházel v letech 1995-2008. ACC JOURNAL is an international scientific journal. It is published by the Technical University of Liberec. Four universities united in the Academic Coordination Centre in the Euroregion Nisa participate in its production. There are usually three issues of the journal annually. ACC JOURNAL is a periodical publishing original reviewed scientific papers, scientific studies, papers presented at conferences, and findings of research projects. The first issue focuses on natural sciences and technology, the second issue deals with the science of economics, and the third issue contains findings from the area of social sciences. ACC JOURNAL is a reviewed one. It is building upon the tradition of the “Scientific Treatises” published between 1995 and 2008. -

2018 FIFA WORLD CUP RUSSIA'n' WATERWAYS
- The 2018 FIFA World Cup will be the 21st FIFA World Cup, a quadrennial international football tournament contested by the men's national teams of the member associations of FIFA. It is scheduled to take place in Russia from 14 June to 15 July 2018,[2] 2018 FIFA WORLD CUP RUSSIA’n’WATERWAYS after the country was awarded the hosting rights on 2 December 2010. This will be the rst World Cup held in Europe since 2006; all but one of the stadium venues are in European Russia, west of the Ural Mountains to keep travel time manageable. - The nal tournament will involve 32 national teams, which include 31 teams determined through qualifying competitions and Routes from the Five Seas 14 June - 15 July 2018 the automatically quali ed host team. A total of 64 matches will be played in 12 venues located in 11 cities. The nal will take place on 15 July in Moscow at the Luzhniki Stadium. - The general visa policy of Russia will not apply to the World Cup participants and fans, who will be able to visit Russia without a visa right before and during the competition regardless of their citizenship [https://en.wikipedia.org/wiki/2018_FIFA_World_Cup]. IDWWS SECTION: Rybinsk – Moscow (433 km) Barents Sea WATERWAYS: Volga River, Rybinskoye, Ughlichskoye, Ivan’kovskoye Reservoirs, Moscow Electronic Navigation Charts for Russian Inland Waterways (RIWW) Canal, Ikshinskoye, Pestovskoye, Klyaz’minskoye Reservoirs, Moskva River 600 MOSCOW Luzhniki Arena Stadium (81.000), Spartak Arena Stadium (45.000) White Sea Finland Belomorsk [White Sea] Belomorsk – Petrozavodsk (402 km) Historic towns: Rybinsk, Ughlich, Kimry, Dubna, Dmitrov Baltic Sea Lock 13,2 White Sea – Baltic Canal, Onega Lake Small rivers: Medveditsa, Dubna, Yukhot’, Nerl’, Kimrka, 3 Helsinki 8 4,0 Shosha, Mologa, Sutka 400 402 Arkhangel’sk Towns: Seghezha, Medvezh’yegorsk, Povenets Lock 12,2 Vyborg Lakes: Vygozero, Segozero, Volozero (>60.000 lakes) 4 19 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 30 1 2 3 6 7 10 14 15 4,0 MOSCOW, Group stage 1/8 1/4 1/2 3 1 Estonia Petrozavodsk IDWWS SECTION: [Baltic Sea] St. -

Use of Classification Algorithms for the Ice Jams Forecasting Problem
E3S Web of Conferences 163, 02008 (2020) https://doi.org/10.1051/e3sconf/202016302008 IV Vinogradov Conference Use of classification algorithms for the ice jams forecasting problem Natalia Semenova1*, Alexey Sazonov1,2, Inna Krylenko1,2,andNatalia Frolova1 1 Lomonosov Moscow State University, GSP-1, Leninskie Gory, 119991, Moscow, Russia 2 Water Problems Institute of the Russian Academy of Science, Gubkina st., 3, 119333, Moscow, Russia Abstract. In the research the prediction of occurrence of ice jam based on the K Nearest Neighbor method was considered by example of the city of Velikiy Ustyug, located at the confluence of the Sukhona and Yug Rivers. A forecast accuracy of 82% was achieved based on selected most significant hydrological and meteorological features. 1 Introduction Floods gain a lead among natural disasters both in terms of area of distribution and damage caused for Russia. Flooding can be caused by snow cover melting, a large amount of precipitation, the effects of surges, a breakthrough of a dam, etc. For northern rivers, including rivers of the European part of Russia, ice jams often cause floods. The goal of this research is developing a methodology for predicting the occurrence of ice jam based on the machine learning method. The place of confluence of the Sukhona and Yug Rivers, where the city of Velikiy Ustyug is located, was chosen as the object of study. The probability of the ice jams formation in this area is 43.5% according to statistics. Their occurrence leads to an increase of water level and flooding of residential and utility buildings. 2 Data and methods Over the past two decades, there has been a huge leap in the development of computer technology and machine learning, which has allowed the application of various machine learning algorithms to a large number of applied problems, including the prediction of flood characteristics. -

Nikolai Klyuev
Nikolai Klyuev Nikolai Klyuev (1884-1937) is one of the most intriguing figures in Russian Modernism. A “new-peasant poet” from the Russian north, but also a product of and producer of Russian Modernist poetry; a cultural archaist, but also a homosexual; a promoter of the values and interests both of the Old Belief, and of Russian sectarianism, but also, especially in his later years, a defender of Orthodoxy; an erstwhile member of the Bolshevik Party, who was arrested and exiled in 1934, and finally (and predictably) arrested and shot in 1937; a poet who self-consciously promoted himself as a “prophet”, some of whose prophetic verses have indeed proved remarkably accurate (ecological problems for Russia, disaster from Bolshevik policies, his own recovery from oblivion, and so on) – he is a complex and fascinatingly contradictory figure. His poetry, moreover, is both difficult and intriguing. An excellent Russian-language site (with minor contributions from M. Makin, it should be admitted) devoted to all the “new-peasant poets” can be found at: Новокрестьянские поэты. There you will find more of Klyuev’s poetry, and a lot of other material on the poet. An even Russian better site, devoted entirely to the poet is Сайт, посвященный изучению творчества Николая Клюева Brief Chronology 1884 Born 10 October, village of Koshtugi, on river Megra, southern Vytegra uezd (district), Olonia Guberniya (province; present day Vytegra region, Vologda oblast’). Father, Nikolai Timofeevich (1842-1918) village police constable, former soldier. Mother, Praskov’ya Dmitrievna (1851-1913), said to have been very religious. Klyuev’s claims to literal descent from Old Believers (dissenters from official Orthodoxy, who refuse to accept the church reforms of the 17th century and the social reforms of the Petrine era) are always through the maternal line. -

Economic and Social Changes: Facts, Trends, Forecast
THE RUSSIAN ACADEMY OF SCIENCES INSTITUTE OF SOCIO-ECONOMIC DEVELOPMENT OF TERRITORIES OF RAS ECONOMIC AND SOCIAL CHANGES: FACTS, TRENDS, FORECAST 6 (36) 2014 The journal is published according to the decision of RAS economic institutions’ administration in the Northwestern Federal District Institute of Economics of Karelian Scientific Centre of RAS (Karelia Republic) G.P. Luzin Institute of Economic Problems of Kola Scientific Centre of RAS (Murmansk Oblast) Institute of Socio-Economic Development of Territories of RAS (Vologda Oblast) and according to the decision of the administration of Saint Petersburg State University of Economics and Finance Cherepovets State University (Vologda Oblast) and RAS institutions of other RF regions Institute of Social and Economic Research of Ufa Science Centre of RAS (Bashkortostan Republic) Institute of Economics of the Ural RAS Department (Sverdlovsk Oblast) The decision of Presidium of the Higher Attestation Commission of the Russian MES (No.6/6, dated 19.02.2010) the journal is included in the list of leading scientific editions, recommended for publication of the main results of dissertations for the degree of Doctor and Candidate of Sciences. The journal is included into databases: VINITI RAS, Ulrich's Periodicals Directory, Index Copernicus International, EBSCOhost, Proquest, and also into the Russian Science Citation Index, and is presented in the open access on the platform of the Scientific e-Library (http://www. elibrary.ru). In 2014 the German National Library of Economics included the Journal into its fund. The journal is also sent to the Library of Congress, the USA. All research articles submitted to the Journal are subject to mandatory peer-review. -

Subject of the Russian Federation)
How to use the Atlas The Atlas has two map sections The Main Section shows the location of Russia’s intact forest landscapes. The Thematic Section shows their tree species composition in two different ways. The legend is placed at the beginning of each set of maps. If you are looking for an area near a town or village Go to the Index on page 153 and find the alphabetical list of settlements by English name. The Cyrillic name is also given along with the map page number and coordinates (latitude and longitude) where it can be found. Capitals of regions and districts (raiony) are listed along with many other settlements, but only in the vicinity of intact forest landscapes. The reader should not expect to see a city like Moscow listed. Villages that are insufficiently known or very small are not listed and appear on the map only as nameless dots. If you are looking for an administrative region Go to the Index on page 185 and find the list of administrative regions. The numbers refer to the map on the inside back cover. Having found the region on this map, the reader will know which index map to use to search further. If you are looking for the big picture Go to the overview map on page 35. This map shows all of Russia’s Intact Forest Landscapes, along with the borders and Roman numerals of the five index maps. If you are looking for a certain part of Russia Find the appropriate index map. These show the borders of the detailed maps for different parts of the country. -
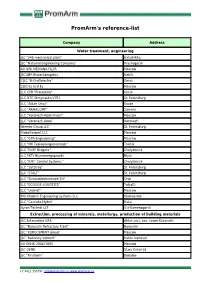
Promarm's Reference-List
PromArm's reference-list Company Address Water treatment, engineering JSC "345 mechanical plant" Balashikha JSC "National Engineering Company" Krasnogorsk AO NPK MEDIANA-FILTR Moscow JSC NPP Biotechprogress Kirishi CJSC "B-Graffelectro" Omsk CJSC Es End Ey Moscow LLC CPB "Protection" Omsk LLC NTC Stroynauka-VITU St. Petersburg LLC "Aidan Stroy" Kazan LLC "ARMACOMP" Samara LLC "Voronezh-Aqua Invest" Moscow LLC "Voronezh-Aqua" Voronezh Hermes Group LLC St. Petersburg Globaltexport LLC Moscow LLC "GPA Engineering" Moscow LLC "MK Teploenergomontazh" Troitsk LLC "NVK" Niagara " Chelyabinsk LLC PKTs Biyskenergoproekt Biysk LLC "RPK" Control Systems " Chelyabinsk LLC "SetStroy" St. Petersburg LLC "STALT" St. Petersburg LLC "Stroisantechservice-1N" Orsk LLC "ECOLINE-LOGISTICS" Tolyatti LLC "Unimet" Moscow PKK Modern Engineering Systems LLC Vladivostok LLC "Cascade-Hydro" Baku Ayron-Technik LLP Ust-Kamenogorsk Extraction, processing of minerals, metallurgy, production of building materials JSC Aldanzoloto GRK Aldan ulus, pos. Lower Kuranakh JSC "Borovichi Refractory Plant" Borovichi JSC "EUROCEMENT group" Moscow JSC "Katavsky cement" Katav-Ivanovsk AO OKHK URALCHEM Moscow JSC OEMK Stary Oskol-15 JSC "Firstborn" Bodaibo +7 8412 350797, [email protected], www.promarm.ru JSC "Aleksandrovsky Mine" Mogochinsky district of Davenda JSC RUSAL Ural Kamensk-Uralsky JSC "SUAL" Kamensk-Uralsky JSC "Khiagda" Bounty district, with. Bagdarin JSC "RUSAL Sayanogorsk" Sayanogorsk CJSC "Karabashmed" Karabash CJSC "Liskinsky gas silicate" Voronezh CJSC "Mansurovsky career management" Istra district, Alekseevka village Mineralintech CJSC Norilsk JSC "Oskolcement" Stary Oskol CJSC RCI Podolsk Refractories Shcherbinka Bonolit OJSC - Construction Solutions Old Kupavna LLC "AGMK" Amursk LLC "Borgazobeton" Boron Volga Cement LLC Nizhny Novgorod LLC "VOLMA-Absalyamovo" Yutazinsky district, with. Absalyamovo LLC "VOLMA-Orenburg" Belyaevsky district, pos. -

The Periglacial Climate and Environment in Northern Eurasia
ARTICLE IN PRESS Quaternary Science Reviews 23 (2004) 1333–1357 The periglacial climate andenvironment in northern Eurasia during the Last Glaciation Hans W. Hubbertena,*, Andrei Andreeva, Valery I. Astakhovb, Igor Demidovc, Julian A. Dowdeswelld, Mona Henriksene, Christian Hjortf, Michael Houmark-Nielseng, Martin Jakobssonh, Svetlana Kuzminai, Eiliv Larsenj, Juha Pekka Lunkkak, AstridLys a(j, Jan Mangerude, Per Moller. f, Matti Saarnistol, Lutz Schirrmeistera, Andrei V. Sherm, Christine Siegerta, Martin J. Siegertn, John Inge Svendseno a Alfred Wegener Institute for Polar and Marine Research (AWI), Telegrafenberg A43, Potsdam D-14473, Germany b Geological Faculty, St. Petersburg University, Universitetskaya 7/9, St. Petersburg 199034, Russian Federation c Institute of Geology, Karelian Branch of Russian Academy of Sciences, Pushkinskaya 11, Petrozavodsk 125610, Russian Federation d Scott Polar Research Institute and Department of Geography, University of Cambridge, Cambridge CBZ IER, UK e Department of Earth Science, University of Bergen, Allegt.! 41, Bergen N-5007, Norway f Quaternary Science, Department of Geology, Lund University, Geocenter II, Solvegatan. 12, Lund Sweden g Geological Institute, University of Copenhagen, Øster Voldgade 10, Copenhagen DK-1350, Denmark h Center for Coastal and Ocean Mapping, Chase Ocean Engineering Lab, University of New Hampshire, Durham, NH 03824, USA i Paleontological Institute, RAS, Profsoyuznaya ul., 123, Moscow 117868, Russia j Geological Survey of Norway, PO Box 3006 Lade, Trondheim N-7002, Norway