The Crystal Structure of Minerals - H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crystal Structures
Crystal Structures Academic Resource Center Crystallinity: Repeating or periodic array over large atomic distances. 3-D pattern in which each atom is bonded to its nearest neighbors Crystal structure: the manner in which atoms, ions, or molecules are spatially arranged. Unit cell: small repeating entity of the atomic structure. The basic building block of the crystal structure. It defines the entire crystal structure with the atom positions within. Lattice: 3D array of points coinciding with atom positions (center of spheres) Metallic Crystal Structures FCC (face centered cubic): Atoms are arranged at the corners and center of each cube face of the cell. FCC continued Close packed Plane: On each face of the cube Atoms are assumed to touch along face diagonals. 4 atoms in one unit cell. a 2R 2 BCC: Body Centered Cubic • Atoms are arranged at the corners of the cube with another atom at the cube center. BCC continued • Close Packed Plane cuts the unit cube in half diagonally • 2 atoms in one unit cell 4R a 3 Hexagonal Close Packed (HCP) • Cell of an HCP lattice is visualized as a top and bottom plane of 7 atoms, forming a regular hexagon around a central atom. In between these planes is a half- hexagon of 3 atoms. • There are two lattice parameters in HCP, a and c, representing the basal and height parameters Volume respectively. 6 atoms per unit cell Coordination number – the number of nearest neighbor atoms or ions surrounding an atom or ion. For FCC and HCP systems, the coordination number is 12. For BCC it’s 8. -

Glide and Screw
Space Groups •The 32 crystallographic point groups, whose operation have at least one point unchanged, are sufficient for the description of finite, macroscopic objects. •However since ideal crystals extend indefinitely in all directions, we must also include translations (the Bravais lattices) in our description of symmetry. Space groups: formed when combining a point symmetry group with a set of lattice translation vectors (the Bravais lattices), i.e. self-consistent set of symmetry operations acting on a Bravais lattice. (Space group lattice types and translations have no meaning in point group symmetry.) Space group numbers for all the crystal structures we have discussed this semester, and then some, are listed in DeGraef and Rohrer books and pdf. document on structures and AFLOW website, e.g. ZnS (zincblende) belongs to SG # 216: F43m) Class21/1 Screw Axes •The combination of point group symmetries and translations also leads to two additional operators known as glide and screw. •The screw operation is a combination of a rotation and a translation parallel to the rotation axis. •As for simple rotations, only diad, triad, tetrad and hexad axes, that are consistent with Bravais lattice translation vectors can be used for a screw operator. •In addition, the translation on each rotation must be a rational fraction of the entire translation. •There is no combination of rotations or translations that can transform the pattern produced by 31 to the pattern of 32 , and 41 to the pattern of 43, etc. •Thus, the screw operation results in handedness Class21/2 or chirality (can’t superimpose image on another, e.g., mirror image) to the pattern. -

Crystal Structure of a Material Is Way in Which Atoms, Ions, Molecules Are Spatially Arranged in 3-D Space
Crystalline Structures – The Basics •Crystal structure of a material is way in which atoms, ions, molecules are spatially arranged in 3-D space. •Crystal structure = lattice (unit cell geometry) + basis (atom, ion, or molecule positions placed on lattice points within the unit cell). •A lattice is used in context when describing crystalline structures, means a 3-D array of points in space. Every lattice point must have identical surroundings. •Unit cell: smallest repetitive volume •Each crystal structure is built by stacking which contains the complete lattice unit cells and placing objects (motifs, pattern of a crystal. A unit cell is chosen basis) on the lattice points: to represent the highest level of geometric symmetry of the crystal structure. It’s the basic structural unit or building block of crystal structure. 7 crystal systems in 3-D 14 crystal lattices in 3-D a, b, and c are the lattice constants 1 a, b, g are the interaxial angles Metallic Crystal Structures (the simplest) •Recall, that a) coulombic attraction between delocalized valence electrons and positively charged cores is isotropic (non-directional), b) typically, only one element is present, so all atomic radii are the same, c) nearest neighbor distances tend to be small, and d) electron cloud shields cores from each other. •For these reasons, metallic bonding leads to close packed, dense crystal structures that maximize space filling and coordination number (number of nearest neighbors). •Most elemental metals crystallize in the FCC (face-centered cubic), BCC (body-centered cubic, or HCP (hexagonal close packed) structures: Room temperature crystal structure Crystal structure just before it melts 2 Recall: Simple Cubic (SC) Structure • Rare due to low packing density (only a-Po has this structure) • Close-packed directions are cube edges. -

C:\Documents and Settings\Alan Smithee\My Documents\MOTM
I`mt`qx1/00Lhmdq`knesgdLnmsg9Rbnkdbhsd This month’s mineral, scolecite, is an uncommon zeolite from India. Our write-up explains its origin as a secondary mineral in volcanic host rocks, the difficulty of collecting this fragile mineral, the unusual properties of the zeolite-group minerals, and why mineralogists recently revised the system of zeolite classification and nomenclature. OVERVIEW PHYSICAL PROPERTIES Chemistry: Ca(Al2Si3O10)A3H2O Hydrous Calcium Aluminum Silicate (Hydrous Calcium Aluminosilicate), usually containing some potassium and sodium. Class: Silicates Subclass: Tectosilicates Group: Zeolites Crystal System: Monoclinic Crystal Habits: Usually as radiating sprays or clusters of thin, acicular crystals or Hairlike fibers; crystals are often flattened with tetragonal cross sections, lengthwise striations, and slanted terminations; also massive and fibrous. Twinning common. Color: Usually colorless, white, gray; rarely brown, pink, or yellow. Luster: Vitreous to silky Transparency: Transparent to translucent Streak: White Cleavage: Perfect in one direction Fracture: Uneven, brittle Hardness: 5.0-5.5 Specific Gravity: 2.16-2.40 (average 2.25) Figure 1. Scolecite. Luminescence: Often fluoresces yellow or brown in ultraviolet light. Refractive Index: 1.507-1.521 Distinctive Features and Tests: Best field-identification marks are acicular crystal habit; vitreous-to-silky luster; very low density; and association with other zeolite-group minerals, especially the closely- related minerals natrolite [Na2(Al2Si3O10)A2H2O] and mesolite [Na2Ca2(Al6Si9O30)A8H2O]. Laboratory tests are often needed to distinguish scolecite from other zeolite minerals. Dana Classification Number: 77.1.5.5 NAME The name “scolecite,” pronounced SKO-leh-site, is derived from the German Skolezit, which comes from the Greek sklx, meaning “worm,” an allusion to the tendency of its acicular crystals to curl when heated and dehydrated. -

Types of Lattices
Types of Lattices Types of Lattices Lattices are either: 1. Primitive (or Simple): one lattice point per unit cell. 2. Non-primitive, (or Multiple) e.g. double, triple, etc.: more than one lattice point per unit cell. Double r2 cell r1 r2 Triple r1 cell r2 r1 Primitive cell N + e 4 Ne = number of lattice points on cell edges (shared by 4 cells) •When repeated by successive translations e =edge reproduce periodic pattern. •Multiple cells are usually selected to make obvious the higher symmetry (usually rotational symmetry) that is possessed by the 1 lattice, which may not be immediately evident from primitive cell. Lattice Points- Review 2 Arrangement of Lattice Points 3 Arrangement of Lattice Points (continued) •These are known as the basis vectors, which we will come back to. •These are not translation vectors (R) since they have non- integer values. The complexity of the system depends upon the symmetry requirements (is it lost or maintained?) by applying the symmetry operations (rotation, reflection, inversion and translation). 4 The Five 2-D Bravais Lattices •From the previous definitions of the four 2-D and seven 3-D crystal systems, we know that there are four and seven primitive unit cells (with 1 lattice point/unit cell), respectively. •We can then ask: can we add additional lattice points to the primitive lattices (or nets), in such a way that we still have a lattice (net) belonging to the same crystal system (with symmetry requirements)? •First illustrate this for 2-D nets, where we know that the surroundings of each lattice point must be identical. -

The Metrical Matrix in Teaching Mineralogy G. V
THE METRICAL MATRIX IN TEACHING MINERALOGY G. V. GIBBS Department of Geological Sciences & Department of Materials Science and Engineering Virginia Polytechnic Institute and State University Blacksburg, VA 24061 [email protected] INTRODUCTION The calculation of the d-spacings, the angles between planes and zones, the bond lengths and angles and other important geometric relationships for a mineral can be a tedious task both for the student and the instructor, particularly when completed with the large as- sortment of trigonometric identities and algebraic formulae that are available (d. Crystal Geometry (1959), Donnay and Donnay, International Tables for Crystallography, Vol. II, Section 3, The Kynoch Press, 101-158). However, such calculations are straightforward and relatively easy to do when completed with the metrical matrix and the interactive software MATOP. Several applications of the matrix are presented below, each of which is worked out in detail and which is designed to teach you its use in the study of crystal geometry. SOME PRELIMINARY COMMENTS We begin our discussion of the matrix with a brief examination of the properties of the geometric three dimensional space, S, in which we live and in which minerals and rocks occur. For our purposes, it will be convenient to view S as the set of all vectors that radiate from a common origin to each point in space. In constructing a model for S, we chose three noncoplanar, coordinate axes denoted X, Y and Z, each radiating from the origin, O. Next, we place three nonzero vectors denoted a, band c along X, Y and Z, respectively, likewise radiating from O. -
Primitive Cell Wigner-Seitz Cell (WS) Primitive Cell
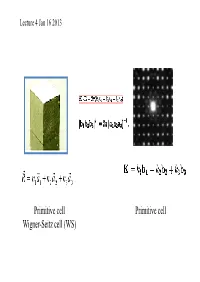
Lecture 4 Jan 16 2013 Primitive cell Primitive cell Wigner-Seitz cell (WS) First Brillouin zone The Wigner-Seitz primitive cell of the reciprocal lattice is known as the first Brillouin zone. (Wigner-Seitz is real space concept while Brillouin zone is a reciprocal space idea). Powder cell Polymorphic Forms of Carbon Graphite – a soft, black, flaky solid, with a layered structure – parallel hexagonal arrays of carbon atoms – weak van der Waal’s forces between layers – planes slide easily over one another Miller indices Simple cubic Miller Indices Rules for determining Miller Indices: 1. Determine the intercepts of the face along the crystallographic axes, in terms of un it ce ll dimens ions. 2. Take the reciprocals 3. Clear fractions 4. Reduce to lowest terms Simple cubic d100=? Where does a protein crystallographer see the Miller indices? CtlCommon crystal faces are parallel to lattice planes • Eac h diffrac tion spo t can be regarded as a X-ray beam reflected from a lattice plane , and therefore has a unique Miller index. Miller indices A Miller index is a series of coprime integers that are inversely ppproportional to the intercepts of the cry stal face or crystallographic planes with the edges of the unit cell. It describes the orientation of a plane in the 3-D lattice with respect to the axes. The general form of the Miller index is (h, k, l) where h, k, and l are integers related to the unit cell along the a, b, c crystal axes. Irreducible brillouin zone II II II II Reciprocal lattice ghakblc The Bravais lattice after Fourier transform real space reciprocal lattice normaltthlls to the planes (vect ors ) poitints spacing between planes 1/distance between points ((y,p)actually, 2p/distance) l (distance, wavelength) 2p/l=k (momentum, wave number) BillBravais cell Wigner-SiSeitz ce ll Brillouin zone . -

(M = Ca, Mg, Fe2+), a Structural Base of Ca3mg3(PO4)4 Phosphors
crystals Article Crystal Chemistry of Stanfieldite, Ca7M2Mg9(PO4)12 (M = Ca, Mg, Fe2+), a Structural Base of Ca3Mg3(PO4)4 Phosphors Sergey N. Britvin 1,2,* , Maria G. Krzhizhanovskaya 1, Vladimir N. Bocharov 3 and Edita V. Obolonskaya 4 1 Department of Crystallography, Institute of Earth Sciences, St. Petersburg State University, Universitetskaya Nab. 7/9, 199034 St. Petersburg, Russia; [email protected] 2 Nanomaterials Research Center, Kola Science Center of Russian Academy of Sciences, Fersman Str. 14, 184209 Apatity, Russia 3 Centre for Geo-Environmental Research and Modelling, Saint-Petersburg State University, Ulyanovskaya ul. 1, 198504 St. Petersburg, Russia; [email protected] 4 The Mining Museum, Saint Petersburg Mining University, 2, 21st Line, 199106 St. Petersburg, Russia; [email protected] * Correspondence: [email protected] Received: 1 May 2020; Accepted: 25 May 2020; Published: 1 June 2020 Abstract: Stanfieldite, natural Ca-Mg-phosphate, is a typical constituent of phosphate-phosphide assemblages in pallasite and mesosiderite meteorites. The synthetic analogue of stanfieldite is used as a crystal matrix of luminophores and frequently encountered in phosphate bioceramics. However, the crystal structure of natural stanfieldite has never been reported in detail, and the data available so far relate to its synthetic counterpart. We herein provide the results of a study of stanfieldite from the Brahin meteorite (main group pallasite). The empirical formula of the mineral is Ca8.04Mg9.25Fe0.72Mn0.07P11.97O48. Its crystal structure has been solved and refined to R1 = 0.034. Stanfieldite from Brahin is monoclinic, C2/c, a 22.7973(4), b 9.9833(2), c 17.0522(3) Å, β 99.954(2)◦, 3 V 3822.5(1)Å . -

Research on Crystal Growth and Characterization at the National Bureau of Standards January to June 1964
NATL INST. OF STAND & TECH R.I.C AlllDS bnSflb *^,; National Bureau of Standards Library^ 1*H^W. Bldg Reference book not to be '^sn^ t-i/or, from the library. ^ecknlccil v2ote 251 RESEARCH ON CRYSTAL GROWTH AND CHARACTERIZATION AT THE NATIONAL BUREAU OF STANDARDS JANUARY TO JUNE 1964 U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS tiona! Bureau of Standards NOV 1 4 1968 151G71 THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. Its responsibilities include development and maintenance of the national stand- ards of measurement, and the provisions of means for making measurements consistent with those standards; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be necessary, particu- larly for government agencies; cooperation in the establishment of standard practices for incorpora- tion in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of devices to serve special needs of the Government; assistance to industry, business, and consumers in the development and acceptance of commercial standards and simplified trade practice recommendations; administration of programs in cooperation with United States business groups and standards organizations for the development of international standards of practice; and maintenance of a clearinghouse for the collection and dissemination of scientific, tech- nical, and engineering information. The scope of the Bureau's activities is suggested in the following listing of its four Institutes and their organizational units. -

Space Symmetry, Space Groups
Space symmetry, Space groups - Space groups are the product of possible combinations of symmetry operations including translations. - There exist 230 different space groups in 3-dimensional space - Comparing to point groups, space groups have 2 more symmetry operations (table 1). These operations include translations. Therefore they describe not only the lattice but also the crystal structure. Table 1: Additional symmetry operations in space symmetry, their description, symmetry elements and Hermann-Mauguin symbols. symmetry H-M description symmetry element operation symbol screw 1. rotation by 360°/N screw axis NM (Schraubung) 2. translation along the axis (Schraubenachse) 1. reflection across the plane glide glide plane 2. translation parallel to the a,b,c,n,d (Gleitspiegelung) (Gleitspiegelebene) glide plane Glide directions for the different gilde planes: a (b,c): translation along ½ a or ½ b or ½ c, respectively) (with ,, cba = vectors of the unit cell) n: translation e.g. along ½ (a + b) d: translation e.g. along ¼ (a + b), d from diamond, because this glide plane occurs in the diamond structure Screw axis example: 21 (N = 2, M = 1) 1) rotation by 180° (= 360°/2) 2) translation parallel to the axis by ½ unit (M/N) Only 21, (31, 32), (41, 43), 42, (61, 65) (62, 64), 63 screw axes exist in parenthesis: right, left hand screw axis Space group symbol: The space group symbol begins with a capital letter (P: primitive; A, B, C: base centred I, body centred R rhombohedral, F face centred), which represents the Bravais lattice type, followed by the short form of symmetry elements, as known from the point group symbols. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Apophyllite-(Kf)
December 2013 Mineral of the Month APOPHYLLITE-(KF) Apophyllite-(KF) is a complex mineral with the unusual tendency to “leaf apart” when heated. It is a favorite among collectors because of its extraordinary transparency, bright luster, well- developed crystal habits, and occurrence in composite specimens with various zeolite minerals. OVERVIEW PHYSICAL PROPERTIES Chemistry: KCa4Si8O20(F,OH)·8H20 Basic Hydrous Potassium Calcium Fluorosilicate (Basic Potassium Calcium Silicate Fluoride Hydrate), often containing some sodium and trace amounts of iron and nickel. Class: Silicates Subclass: Phyllosilicates (Sheet Silicates) Group: Apophyllite Crystal System: Tetragonal Crystal Habits: Usually well-formed, cube-like or tabular crystals with rectangular, longitudinally striated prisms, square cross sections, and steep, diamond-shaped, pyramidal termination faces; pseudo-cubic prisms usually have flat terminations with beveled, distinctly triangular corners; also granular, lamellar, and compact. Color: Usually colorless or white; sometimes pale shades of green; occasionally pale shades of yellow, red, blue, or violet. Luster: Vitreous to pearly on crystal faces, pearly on cleavage surfaces with occasional iridescence. Transparency: Transparent to translucent Streak: White Cleavage: Perfect in one direction Fracture: Uneven, brittle. Hardness: 4.5-5.0 Specific Gravity: 2.3-2.4 Luminescence: Often fluoresces pale yellow-green. Refractive Index: 1.535-1.537 Distinctive Features and Tests: Pseudo-cubic crystals with pearly luster on cleavage surfaces; longitudinal striations; and occurrence as a secondary mineral in association with various zeolite minerals. Laboratory analysis is necessary to differentiate apophyllite-(KF) from closely-related apophyllite-(KOH). Can be confused with such zeolite minerals as stilbite-Ca [hydrous calcium sodium potassium aluminum silicate, Ca0.5,K,Na)9(Al9Si27O72)·28H2O], which forms tabular, wheat-sheaf-like, monoclinic crystals.