Structure Determination of Gaseous and Amorphous Substances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemistry News
Fall 2015 Chemistry News Filling the Chemical Sciences Pipeline Future chemists are being recruited by the Department of Chemistry at every age from grade school children to postdoctoral scholars. Hands-on activities encourage girls to learn and explore their potential in science, technology, math and engineering. FEMMES, a UM student organization, organizes events for 4th-6th grade girls on campus and in afterschool programs. Chemistry faculty organized lab tours for students students newly admitted to the University of Michigan in the Summer Bridge program. afety glasses and gloves in place, Johana and a dozen other fourth grade Sgirls used pipettes to drip a creamy mixture into cups of liquid nitrogen, watching the drops freeze instantly into an ice cream treat. After the graduate students strained the tasty dots out of the cup, the girls were able to eat the Cass Technical High School students spent the summer doing result of their experiment. research as part of the UM Detroit Research Internship These girls were attending an all-day event at the UM Chemistry build- Summer Experience, D-RISE. They participated alongside the ing organized by FEMMES, Females Excelling More in Math, Engineering, UM graduate students in the Karle Symposium. and the Sciences. FEMMES aims to inspire young girls, encouraging them to consider their potential in science and math. Each event features hands-on activities led by volunteers. Chemistry’s undergraduates, graduate students, and faculty are very active in the Michigan chapter of FEMMES. On a recent Saturday, FEMMES brought 300 girls to campus from Detroit and area schools to participate in twenty different activities. -

Annual Report 2013.Pdf
ATOMIC HERITAGE FOUNDATION Preserving & Interpreting Manhattan Project History & Legacy preserving history ANNUAL REPORT 2013 WHY WE SHOULD PRESERVE THE MANHATTAN PROJECT “The factories and bombs that Manhattan Project scientists, engineers, and workers built were physical objects that depended for their operation on physics, chemistry, metallurgy, and other nat- ural sciences, but their social reality - their meaning, if you will - was human, social, political....We preserve what we value of the physical past because it specifically embodies our social past....When we lose parts of our physical past, we lose parts of our common social past as well.” “The new knowledge of nuclear energy has undoubtedly limited national sovereignty and scaled down the destructiveness of war. If that’s not a good enough reason to work for and contribute to the Manhattan Project’s historic preservation, what would be? It’s certainly good enough for me.” ~Richard Rhodes, “Why We Should Preserve the Manhattan Project,” Bulletin of the Atomic Scientists, May/June 2006 Photographs clockwise from top: J. Robert Oppenheimer, General Leslie R. Groves pinning an award on Enrico Fermi, Leona Woods Marshall, the Alpha Racetrack at the Y-12 Plant, and the Bethe House on Bathtub Row. Front cover: A Bruggeman Ranch property. Back cover: Bronze statues by Susanne Vertel of J. Robert Oppenheimer and General Leslie Groves at Los Alamos. Table of Contents BOARD MEMBERS & ADVISORY COMMITTEE........3 Cindy Kelly, Dorothy and Clay Per- Letter from the President..........................................4 -

ACA Structure Matters, Winter 2017
ACA Isabella Karle (1921-2017) Winter 2017 Structure Matters Remembering Isabella Karle “I need to tackle this scientific problem, what techniques are Isabella Karle (1921 - 2017), retired from the Naval Research available to help me do it?” and when she found out what they Laboratory (Washington, DC) after more than six decades there, were she would solve the problem. In this case she worked with passed away on October 3, 2017, at the age of 95, from a brain silica tubes that she had made, filled with crude plutonium oxide tumor. Early on Isabella was told by a teacher that chemistry and chemical reactants, and inserted them in a hole in a large was not a “proper field for girls” but she went on to become a block of copper that was heated to high temperatures of 800 to member of the National Academy of Sciences. She received the 900 degrees Centigrade. After many experiments under difficult 1988 Gregori Aminoff Prize from the Royal Swedish Academy conditions, she ended up, triumphantly, with bright green crystals of plutonium chloride (PuCl ) that she passed on to the physics of Sciences, the 1993 Bower Award and Prize for Achievement 3 in Science and, in 1995, received the National Medal of Science. branch of the Manhattan project. What follows are remembrances from several of her colleagues. Isabella approached direct methods in the same way, to the delight of her husband Jerry, who won the Nobel Prize for his work on them. She worked hard to find how to run direct methods correctly and then was able to help others. -

SCIENCE HISTORY INSTITUTE ISABELLA KARLE and JEROME
SCIENCE HISTORY INSTITUTE ISABELLA KARLE and JEROME KARLE Transcript of an Interview Conducted by James J. Bohning and David K. Van Keuren at Naval Research Laboratory Washington, District of Columbia on 26 February, 15 June and 9 September 1987 (With Subsequent Corrections and Additions) Upon Isabella Karle’s death in 2017, this oral history was designated Free Access. Please note: This oral history is protected by U.S. copyright law and shall not be reproduced or disseminated in any way without the express permission of the Science History Institute. Users citing this interview for purposes of publication are obliged under the terms of the Center for Oral History, Science History Institute, to credit the Science History Institute using the format below: Isabella Karle and Jerome Karle, interview by James J. Bohning and David K. Van Keuren at Naval Research Laboratory, Washington, District of Columbia, 26 February, 15 June and 9 September 1987 (Philadelphia: Science History Institute, Oral History Transcript # 0066). Formed by the merger of the Chemical Heritage Foundation and the Life Sciences Foundation, the Science History Institute collects and shares the stories of innovators and of discoveries that shape our lives. We preserve and interpret the history of chemistry, chemical engineering, and the life sciences. Headquartered in Philadelphia, with offices in California and Europe, the Institute houses an archive and a library for historians and researchers, a fellowship program for visiting scholars from around the globe, a community of researchers who examine historical and contemporary issues, and an acclaimed museum that is free and open to the public. For more information visit sciencehistory.org. -

PDF File, 2.06 MB
Introduction Have you ever seen those little About the International Year of Crystallography pictures of a molecule of your The United Nations declares 2014 as the official International Year of prescribed medication? …or a drawing Crystallography. It commemorates not only the centennial of X-ray of DNA showing two strands winding Amoxicillin diffraction, which allowed the detailed study of crystalline material, but also the 400th anniversary of Kepler’s observation in 1611 of the What do around each other? symmetrical form of ice crystals, which began the wider study of the role Molecules are too small to be seen by of symmetry in matter. New Drug Design, normal microscopy. Learn more at http://iycr2014.org X-ray crystallography is one of the DNA Studies few techniques that can visualize them About IUCr The International Union of Crystallography is a not-for- and was used to determine the first Schematic picture profit, scientific organization that aims to: and molecular structures ever known. of DNA • promote international cooperation in crystallography • contribute to all aspects of crystallography X-rays X-rays & X-ray Crystallography • promote international publication of crystallographic research have in common? How They Work • facilitate standardization of methods, units, nomenclatures and symbols • form a focus for the relations of crystallography to other sciences • X-ray beams are shot through • We calculate how the diffracted a crystal composed of the X-rays would look, if they The IUCr fulfils these objectives by publishing -
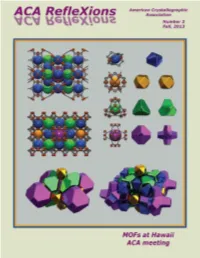
FALL2013.Pdf
SC-XRD_ACA_PrintFP_D8 Quest ECO_Sep 2013 Print.pdf 1 9/3/13 1:22 PM American Crystallographic Association Cover: The cover image, from Omar Farha and Chris Wilmer, Northwestern University, depicts the metal-organic ACA RefleXions framework (MOF), of NU-111. Fall, 2013 See On the Cover, p 3. ACA HOME PAGE: www.AmerCrystalAssn.org Table of Contents 3 President’s Column, On the Cover, Sad News about Dave Rognlie The New D8 QUEST ECO 4 Letter to the Editor, Council Highlights, ACA RefleXions Co-Editors & Staff, Errata Crystallography with a Conscience 6 News & Awards 9 Introducing new staff member Chiara Pastore, 2013 Class of ACA Fellows 10 Contributors to this Issue 13 Net RefleXions, Index of Advertisers 15 Jerome Karle (1918 - 2013) 18 Ray Davis (1938 - 2013) C 20 Charles Norris Caughlan (1915 - 2013), Student Help in Hawaii M Y 21 Poster Prizes in Hawaii CM 24 Accompanying Members MY 25 Annual ACA Meeting in Hawaii CY 64 High School Outreach in Hawaii CMY 65 Books K 66 Workshop on Dynamic Structural Photocrystallography 67 Puzzle Corner 68 46th Course at Erice 71 Bruker/MIT Symposium 72 2014 ACA Meeting in Albuquerque 75 Corporate Members 77 Calendar of Meetings The D8 QUEST ECO Contributions to ACA RefleXions may be sent to either of the Editors: Please address matters pertaining to advertisements, membership With the D8 QUEST ECO, making the right choice is easy. Not only is it affordable and economical inquiries, or use of the ACA mailing list to: with minimal maintenance, it is also environmentally friendly offering low power requirements and Connie (Chidester) Rajnak Judith L. -

The Federal Government: a Nobel Profession
The Federal Government: A Nobel Profession A Report on Pathbreaking Nobel Laureates in Government 1901 - 2002 INTRODUCTION The Nobel Prize is synonymous with greatness. A list of Nobel Prize winners offers a quick register of the world’s best and brightest, whose accomplishments in literature, economics, medicine, science and peace have enriched the lives of millions. Over the past century, 270 Americans have received the Nobel Prize for innovation and ingenuity. Approximately one-fourth of these distinguished individuals are, or were, federal employees. Their Nobel contributions have resulted in the eradication of polio, the mapping of the human genome, the harnessing of atomic energy, the achievement of peace between nations, and advances in medicine that not only prolong our lives, but “This report should serve improve their quality. as an inspiration and a During Public Employees Recognition Week (May 4-10, 2003), in an effort to recognize and honor the reminder to us all of the ideas and accomplishments of federal workers past and present, the Partnership for Public Service offers innovation and nobility of this report highlighting 50 American Nobel laureates the work civil servants do whose award-winning achievements occurred while they served in government or whose public service every day and its far- work had an impact on their career achievements. They were honored for their contributions in the fields reaching impact.” of Physiology or Medicine, Economic Sciences, and Physics and Chemistry. Also included are five Americans whose work merited the Peace Prize. Despite this legacy of accomplishment, too few Americans see the federal government as an incubator for innovation and discovery. -

Nobel Laureates Meet International Top Talents
Nobel Laureates Meet International Top Talents Evaluation Report of the Interdisciplinary Lindau Meeting of Nobel Laureates 2005 Council for the Lindau Nobel Laureate Meetings Foundation Lindau Nobelprizewinners Meetings at Lake Constance Nobel Laureates Meet International Top Talents Evaluation Report of the Interdisciplinary Lindau Meeting of Nobel Laureates 2005 Council for the Lindau Nobel Laureate Meetings Foundation Lindau Nobelprizewinners Meetings at Lake Constance I M PR I N T Published by: Council for the Lindau Nobel Laureate Meetings, Ludwigstr. 68, 88131 Lindau, Germany Foundation Lindau Nobelprizewinners Meetings at Lake Constance, Mainau Castle, 78465 Insel Mainau, Germany Idea and Realisation: Thomas Ellerbeck, Member of the Council for the Lindau Nobel Laureate Meetings and of the Board of Foundation Lindau Nobelprizewinners Meetings at Lake Constance, Alter Weg 100, 32760 Detmold, Germany Science&Media, Büro für Wissenschafts- und Technikkommunikation, Betastr. 9a, 85774 Unterföhring, Germany Texts: Professor Dr. Jürgen Uhlenbusch and Dr. Ulrich Stoll Layout and Production: Vasco Kintzel, Loitersdorf 20a, 85617 Assling, Germany Photos: Peter Badge/typos I, Wrangelstr. 8, 10997 Berlin, Germany Printed by: Druckerei Hermann Brägger, Bankgasse 8, 9001 St. Gallen, Switzerland 2 The Interdisciplinary Lindau Meeting of Nobel Laureates 2005 marked a decisive step for the annual Lindau Meeting in becoming a unique and significant international forum for excellence, fostering the vision of its Spiritus Rector, Count Lennart Bernadotte. It brought together, in the heart of Europe, the world’s current and prospective scientific leaders, the minds that shape the future drive for innovation. As a distinct learning experience, the Meeting stimulated personal dialogue on new discoveries, new methodologies and new issues, as well as on cutting-edge research matters. -

Congressional Record—Senate S6593
July 13, 2000 CONGRESSIONAL RECORD Ð SENATE S6593 E. J. Corey, Harvard University, 1990 Nobel The PRESIDING OFFICER. The Sen- AMENDMENT NO. 3753 Prize in chemistry. ator from Mississippi. Mr. ROCKEFELLER. Mr. President, I James W. Cronin, University of Chicago, Mr. COCHRAN. Mr. President, the am pleased that the Senate has taken 1980 Nobel Prize in physics. an important step toward protecting Renato Dulbecco, The Salk Institute, 1975 Durbin amendment is unnecessary. It Nobel Prize in medicine. purports to direct the manner and de- the lives and property of all Americans Edmond H. Fischer, Univ. of Washington, tails of a missile testing program that with the passage of the Firefighter In- 1992 Nobel Prize in medicine. the Secretary of Defense is committed vestment and Response Enhancement Val L. Fitch, Princeton University, 1980 to conduct already. Act. I am proud today to join with Sen- Nobel Prize in physics. This amendment is an unprecedented ators DODD and DEWINE as a cosponsor Robert F. Furchgott, Suny Health Science effort by the Senate to micromanage a of this legislation. I wish to thank Sen- Ctr., 1998 Nobel Prize in medicine. ator DODD and Senator DEWINE for the Murray Gell-Mann, Santa Fe Institute, weapons system testing program. In no 1969 Nobel Prize in physics. other program has the Senate tried to leadership and effort they have shown Ivar Giaever, Rensselaer Polytechnic Insti- legislate in this way to dictate to DOD on behalf of the men and women serv- tute, 1973 Nobel Prize in physics. how a classified national security test- ing as firefighters across the nation. -

Vintage Munro: Nobel Prize Edition Ebook
VINTAGE MUNRO: NOBEL PRIZE EDITION PDF, EPUB, EBOOK Alice Munro | 224 pages | 04 Aug 2014 | VINTAGE | 9780804173568 | English | United States Vintage Munro: Nobel Prize Edition PDF Book After several funeral services were held and Alfred Nobel's body was cremated, the will was opened. Winners typically are well-versed in their field, with some even taking the extra step to explain their work to nonspecialists and general audiences. Share Flipboard Email. Geology Expert. Camus was French, and so many people assume he was born in France, but he was in fact born, raised, and educated in French Algeria. Mitchell, "for his contribution to the understanding of biological energy transfer through the formulation of the chemiosmotic theory. But even the Vetlesen Prize does not carry Alfred Nobel's charge of conferring "the greatest benefit on mankind. By using ThoughtCo, you accept our. ThoughtCo uses cookies to provide you with a great user experience. The satiric nomination was withdrawn swiftly -- you might even say Jonathan Swift ly. Of those, 10 have been from South Africa, and another six were born in Egypt. The following year, Alfred's younger brother, Emil, was born. As of , not one African Nobel Laureate has been affiliated with an African research institution at the time of their award as determined by the Nobel Prize foundation. Besides being a trained chemist, Alfred was an avid reader of literature and was fluent in English, German, French, Swedish, and Russian. Anfinsen, "for his work on ribonuclease, especially concerning the connection between the amino acid sequence and the biologically active conformation," and Stanford Moore and William H. -

Structure Matters
the protein crystallographer’s ultimate lab automation bundle protein crystallisation 3 instruments year full 2 warranty set of unlimited 1 software licences do you have the steadiest hand in crystallography? take the loop master challenge at ACA 2017! discover more at www.ttplabtech.com fi nd us on ACA - Structure Matters www.AmerCrystalAssn.org Table of Contents 2 President’s Column 2-3 2017 IUCr Meeting & General Assembly Hyderabad 4 New ACA SIG for Cryo-EM 4-5 New ACA SIG for Best Practices for Data Analysis & Archiving 5 What's on the Cover 6-7 News from Canada What's on the Cover Page 5 8 ACA History Site Update 8 Index of Advertisers 9-12 Living History - Alex Wlodawer 13 ACA New Orleans - Workshop on Communication & Innovation 14-15 ACA New Orleans - Workshop on Research Data Management 16 ACA New Orleans - Workshop on Crysalis & OLEX2 16 Contributors to this Issue 18 Spotlight on Stamps 19-20 ACA New Orleans - Travel Grant Recipients 21-23 Obituaries Election Results Isabella Karle (1921-2017) Henry Bragg (1919-2017) 24 News and Awards 24-25 CESTA 2017 - A Study of the Art of Symmetry 26-28 Update on Structural Dynamics 29 ACA Corporate Members 30 Book Reviews 31-33 ACA Elections Results for 2018 34 2017 Contributors to ACA Funds 35 Puzzle Corner 36-37 ACA 2018 NToronto - Preview 38 Call for Nominations 39 ACA 2018 Summer Course in Chemical Crystallography 40 Future Meetings Contributions to ACA RefleXions may be sent to either of the Editors: Please address matters pertaining to advertisements, Judith L. -

OLC Denies FOIA Request for Opinion on Executive Orders
FEDERATION OF AMERICAN SCIENTISTS Board of Sponsors 1725 DeSales Street NW, 6th floor [email protected] (Partial List) Washington, DC 20036 www.fas.org *Sidney Altman Phone: (202) 546-3300 Fax: (202) 675-1010 Bruce Ames F.A.S. *Philip W. Anderson *Kenneth J. Arrow *Julius Axelrod *David Baltimore Frank von Hippel Hal Feiveson Henry C. Kelly Paul Beeson Chairman Secretary-Treasurer President *Baruj Benacerraf *Hans A. Bethe *J. Michael Bishop *Nicolaas Bloembergen *Norman Borlaug *Paul Boyer March 11, 2008 *Owen Chamberlain (202)454-4691 Morris Cohen *Stanley Cohen [email protected] Mildred Cohn *Leon N. Cooper Elizabeth Farris *E. .J. Corey Paul B. Cornely Office of Legal Counsel *James Cronin *Johann Deisenhofer Room 5515, 950 Pennsylvania Avenue, NW Carl Djerassi Ann Druyan Department of Justice *Renato Dulbecco John T. Edsall Washington, DC 20530-0001 Paul R. Ehrlich By fax: 202-514-0563 George Field *Val L. Fitch Jerome D. Frank *Jerome I. Friedman Dear Ms. Farris: *John Kenneth Galbraith *Walter Gilbert *Donald Glaser *Sheldon L. Glashow This is a request under the Freedom of Information Act. Marvin L. Goldberger *Joseph L. Goldstein *Roger C. L. Guillemin We request a copy of an Office of Legal Counsel opinion from the George *Dudley R. Herschbach *Roald Hoffmann W. Bush Administration pertaining in part to the efficacy of executive John P. Holdren *David H. Hubel orders. *Jerome Karle Nathan Keyfitz *H. Gobind Khorana *Arthur Kornberg In particular, Senator Sheldon Whitehouse stated on the Senate floor on *Edwin G. Krebs *Willis E. Lamb December 7 that he had examined an OLC opinion which included, *Leon Lederman *Edward Lewis according to his notes, the following statement or something resembling it: *William N.