Healthpac Formulary Current As of July 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidance on the Use of Mood Stabilizers for the Treatment of Bipolar Affective Disorder Version 2
Guidance on the use of mood stabilizers for the treatment of bipolar affective disorder Version 2 RATIFYING COMMITTEE DRUGS AND THERAPEUTICS GROUP DATE RATIFIED July 2015 REPLACES Version 1 dated July 2013 NEXT REVIEW DATE July 2017 POLICY AUTHORS Jules Haste, Lead Pharmacist, Brighton and Hove Members of the Pharmacy Team (contributors are listed overleaf) If you require this document in an alternative format, i.e. easy read, large text, audio or Braille please contact the pharmacy team on 01243 623349 Page 1 of 49 Contributors Jed Hewitt, Chief Pharmacist - Governance & Professional Practice James Atkinson, Pharmacist Team Leader Mental Health and Community Services Miguel Gomez, Lead Pharmacist, Worthing. Hilary Garforth, Lead Pharmacist, Chichester. Pauline Daw, Lead Pharmacist (CRHTs & AOT), East Sussex. Iftekhar Khan, Lead Pharmacist (S&F Service), East Sussex. Graham Brown, Lead Pharmacist CAMHS & EIS. Gus Fernandez, Specialist Pharmacist MI and MH Lisa Stanton, Specialist Pharmacist Early Intervention Services & Learning Disabilities. Nana Tomova, Specialist Pharmacist, Crawley. Page 2 of 49 Section Title Page Number Introduction and Key Points 4 1. General principles in the treatment of acute mania 6 2. General principles in the treatment of bipolar depression 8 3. General principles in long term treatment 10 4. Rapid cycling 13 5. Physical health 13 6. Treatment in special situations 6.1 Pregnancy 15 6.2 Breast-feeding 17 6.3 Older adults 19 6.4 Children and adolescents 22 6.5 Learning disabilities 29 6.6 Cardiac dysfunction 30 6.7 Renal dysfunction 34 6.8 Hepatic dysfunction 37 6.9 Epilepsy 41 7. The risk of switching to mania with antidepressants 43 8. -

Homicide and Associated Steroid Acute Psychosis: a Case Report
Hindawi Publishing Corporation Case Reports in Medicine Volume 2011, Article ID 564521, 4 pages doi:10.1155/2011/564521 Case Report Homicide and Associated Steroid Acute Psychosis: A Case Report G. Airagnes,1, 2 C. Rouge-Maillart,1, 3 J.-B. Garre,2, 3 and B. Gohier2 1 Service de M´edecine L´egale, CHU d’Angers, 49933 Angers Cedex 09, France 2 D´epartement de Psychiatrie et de Psychologie m´edicale, CHU d’Angers, 49933 Angers Cedex 09, France 3 IFR 132, Universit´e d’Angers, 49035 Angers, France Correspondence should be addressed to G. Airagnes, [email protected] Received 22 June 2011; Revised 26 August 2011; Accepted 26 September 2011 Academic Editor: Massimo Gallerani Copyright © 2011 G. Airagnes et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. We report the case of an old man treated with methylprednisolone for chronic lymphoid leukemia. After two months of treatment, he declared an acute steroid psychosis and beat his wife to death. Steroids were stopped and the psychotic symptoms subsided, but his condition declined very quickly. The clinical course was complicated by a major depressive disorder with suicidal ideas, due to the steroid stoppage, the leukemia progressed, and by a sudden onset of a fatal pulmonary embolism. This clinical case highlights the importance of early detection of steroid psychosis and proposes, should treatment not be stopped, a strategy of dose reduction combined with a mood stabilizer or antipsychotic treatment. -

Appendix D. Study Characteristics
Appendix D. Study Characteristics Table D1. Study characteristics Participant Author Study Study Characteristics Treatment Characteristics Outcomes Reported Characteristics Conclusions Alacqua et al., Recruitment dates: Enrolled: 73 Treatment duration: 3 mo Benefits: NR Adverse events 2008 96 Jan 2002 to Dec 2003 Analyzed: 73 Run-in phase: No occurred frequently Completed: 50 Run-in phase duration: NR Harms: Behavioral during first 3 months Country: Italy Study design: issues, dyskinesia, of treatment with Retrospective cohort GROUP 1 Permitted drugs: NR dystonia, atypical Condition N: 2 dermatologic AE, liver antipsychotics. category: Mixed Diagnostic criteria: Age, mean±SD (range): Prohibited drugs: NR function, hepatic conditions (ADHD, DSM-IV 15.5±0.7 volume, prolactin, ASD, Males %: 50 GROUP 1 prolactin-related AE, schizophrenia- Setting: Caucasian %: NR Drug name: Clozapine sedation, sleepness, related, tics) Outpatient/community Diagnostic breakdown Dosing variability: variable total AE, weight (n): psychosis (1), Target dose (mg/day): NR change Funding: NR Inclusion criteria: (1) schizophrenia (1) Daily dose (mg/day), mean±SD ≤18 yr, (2) received an Treatment naïve (n): all (range): 150±70.1 Newcastle-Ottawa incident treatment with Inpatients (n): NR Concurrent treatments: NR Scale: 6/8 stars atypical antipsychotics First episode psychosis or SSRIs during the (n): NR GROUP 2 study period Comorbidities: NR Drug name: Olanzapine Dosing variability: variable Exclusion criteria: GROUP 2 Target dose (mg/day): NR NR N: 24 Daily dose -

Mechanism of Action of Antidepressants and Mood Stabilizers
79 MECHANISM OF ACTION OF ANTIDEPRESSANTS AND MOOD STABILIZERS ROBERT H. LENOX ALAN FRAZER Bipolar disorder (BPD), the province of mood stabilizers, the more recent understanding that antidepressants share has long been considered a recurrent disorder. For more this property in UPD have focused research on long-term than 50 years, lithium, the prototypal mood stabilizer, has events, such as alterations in gene expression and neuroplas- been known to be effective not only in acute mania but ticity, that may play a significant role in stabilizing the clini- also in the prophylaxis of recurrent episodes of mania and cal course of an illness. In our view, behavioral improvement depression. By contrast, the preponderance of past research and stabilization stem from the acute pharmacologic effects in depression has focused on the major depressive episode of antidepressants and mood stabilizers; thus, both the acute and its acute treatment. It is only relatively recently that and longer-term pharmacologic effects of both classes of investigators have begun to address the recurrent nature of drugs are emphasized in this chapter. unipolar disorder (UPD) and the prophylactic use of long- term antidepressant treatment. Thus, it is timely that we address in a single chapter the most promising research rele- vant to the pharmacodynamics of both mood stabilizers and MOOD STABILIZERS antidepressants. As we have outlined in Fig. 79.1, it is possible to charac- The term mood stabilizer within the clinical setting is com- terize both the course and treatment of bipolar and unipolar monly used to refer to a class of drugs that treat BPD. -

Page 1 of 85 Reference ID: 3091122
HIGHLIGHTS OF PRESCRIBING INFORMATION As an adjunct to 2-5 mg 5-10 mg 15 mg These highlights do not include all the information needed to use ABILIFY antidepressants for the /day /day /day safely and effectively. See full prescribing information for ABILIFY. treatment of major ® ABILIFY (aripiprazole) Tablets depressive disorder – adults ® ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets (2.3) ® Irritability associated with 2 mg/day 5-10 mg/day 15 mg/day ABILIFY (aripiprazole) Oral Solution ® autistic disorder – pediatric ABILIFY (aripiprazole) Injection FOR INTRAMUSCULAR USE ONLY patients (2.4) Initial U.S. Approval: 2002 Agitation associated with 9.75 mg /1.3 30 mg/day schizophrenia or bipolar mL injected injected WARNINGS: INCREASED MORTALITY IN ELDERLY mania – adults (2.5) IM IM PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and • Oral formulations: Administer once daily without regard to meals (2) SUICIDALITY AND ANTIDEPRESSANT DRUGS • IM injection: Wait at least 2 hours between doses. Maximum daily dose See full prescribing information for complete boxed warning. 30 mg (2.5) • Elderly patients with dementia-related psychosis treated with ----------------------DOSAGE FORMS AND STRENGTHS--------------------- antipsychotic drugs are at an increased risk of death. ABILIFY is not approved for the treatment of patients with dementia-related • Tablets: 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, and 30 mg (3) psychosis. (5.1) • Orally Disintegrating Tablets: 10 mg and 15 mg (3) • Oral Solution: 1 mg/mL (3) • Children, adolescents, and young adults taking antidepressants for • Injection: 9.75 mg/1.3 mL single-dose vial (3) major depressive disorder (MDD) and other psychiatric disorders are at increased risk of suicidal thinking and behavior. -
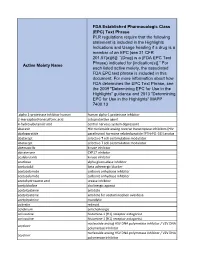
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Mood Stabilizers
Mood Stabilizers Anticonvulsants Depakene/Stavzor (valproic acid) and Depakote/Depakote ER (divalproex sodium) Equetro, Tegretol, and Tegretol-XR (carbamazepine) Lamictal (lamotrigine) Lyrica (pregabalin) Neurontin (gabapentin) Topamax (topiramate) Trileptal (oxcarbazepine) Lithium Carbonate and Lithium Citrate Lithium carbonate Lithium Citrate Lithobid Second-Generation Antipsychotics Abilify (aripiprazole) Clozaril (clozapine) Geodon (ziprasidone) Invega (paliperidone) Risperdal, Risperdal M-Tab, and Risperdal Consta (risperidone) Seroquel (quetiapine) Zyprexa and Zyprexa Zydis (olanzapine) Simply defined, mood stabilizers are medicines used in treating mood disorders such as bipolar disorder and depression. Bipolar disorder is characterized by mood swings in which the individual cycles between mania and depression. The symptoms fluctuate from euphoria and limitless energy in the manic phase to the depths of depression with little energy, guilt, sadness, decreased concentration, lack of appetite, and sleep distur- bance. Because of the wide range of symptoms, from mania to depression, bipolar disorder is often called manic depression. Mood stabilizers are used to treat acute mania, hypomania (a mild form of mania), mixed episodes (when mania and depression coexist in an episode), and depression. Following the acute episode, mood stabilizers Page 2 of 3 MOOD STABILIZERS are used in maintenance therapy to prevent the cyclical relapse of abnormal mood elevations and depressions. The goals of maintenance treatment with a mood stabilizer are to 1) reduce residual symptoms, 2) prevent manic or depressive relapse, 3) reduce the frequency of cycling into the next manic or depressive episode, 4) improve functioning, and 5) reduce the risk of suicide. Lithium Lithium was one of the first mood stabilizers used in the treatment of bipolar disorder. -

A History of the Pharmacological Treatment of Bipolar Disorder
International Journal of Molecular Sciences Review A History of the Pharmacological Treatment of Bipolar Disorder Francisco López-Muñoz 1,2,3,4,* ID , Winston W. Shen 5, Pilar D’Ocon 6, Alejandro Romero 7 ID and Cecilio Álamo 8 1 Faculty of Health Sciences, University Camilo José Cela, C/Castillo de Alarcón 49, 28692 Villanueva de la Cañada, Madrid, Spain 2 Neuropsychopharmacology Unit, Hospital 12 de Octubre Research Institute (i+12), Avda. Córdoba, s/n, 28041 Madrid, Spain 3 Portucalense Institute of Neuropsychology and Cognitive and Behavioural Neurosciences (INPP), Portucalense University, R. Dr. António Bernardino de Almeida 541, 4200-072 Porto, Portugal 4 Thematic Network for Cooperative Health Research (RETICS), Addictive Disorders Network, Health Institute Carlos III, MICINN and FEDER, 28029 Madrid, Spain 5 Departments of Psychiatry, Wan Fang Medical Center and School of Medicine, Taipei Medical University, 111 Hsin Long Road Section 3, Taipei 116, Taiwan; [email protected] 6 Department of Pharmacology, Faculty of Pharmacy, University of Valencia, Avda. Vicente Andrés, s/n, 46100 Burjassot, Valencia, Spain; [email protected] 7 Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Complutense University, Avda. Puerta de Hierro, s/n, 28040 Madrid, Spain; [email protected] 8 Department of Biomedical Sciences (Pharmacology Area), Faculty of Medicine and Health Sciences, University of Alcalá, Crta. de Madrid-Barcelona, Km. 33,600, 28871 Alcalá de Henares, Madrid, Spain; [email protected] * Correspondence: fl[email protected] or [email protected] Received: 4 June 2018; Accepted: 13 July 2018; Published: 23 July 2018 Abstract: In this paper, the authors review the history of the pharmacological treatment of bipolar disorder, from the first nonspecific sedative agents introduced in the 19th and early 20th century, such as solanaceae alkaloids, bromides and barbiturates, to John Cade’s experiments with lithium and the beginning of the so-called “Psychopharmacological Revolution” in the 1950s. -

West Virginia Medicaid
West Virginia Medicaid Bipolar Disorder Management Educational RetroDUR Initial Study Follow – up /Restudy Mailing Executive Summary Purpose: To promote safe and effective drug therapy in patients with Bipolar Disorder (BD). The Veterans Affairs Guidelines, the Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines, and the British Association for Psychopharmacology (BAP) guideline for the management of bipolar disorder provide the foundation for this proposal.1-3 These guidelines, along with recently published major studies, provide performance indicators to evaluate the medication management of BD. Why Issue was Selected: BD is a complex, long-term illness characterized by a repeating course of relapse and remission. It has a lifetime prevalence of 1% to 2% and has been estimated to lead to 2% of all disability-adjusted life-years associated with non-communicable diseases.4,5 Pharmacotherapy is the mainstay of treatment for BD, and treatment guidelines recommend such regimens be built around adequate dosing of a mood stabilizer.1-3 Additional medication classes commonly employed include antipsychotic agents, antidepressants, and stimulants. The principles to effectively employ and combine these different medication groups to obtain optimal outcomes with minimal risks are the focus of this intervention. Program Specific Performance Indicators Candidates Information: Use of an antidepressant in the absence of a mood TBD stabilizer/atypical antipsychotic Extended use of an antidepressant medication TBD Use of multiple atypical antipsychotics simultaneously as TBD mood stabilizers Use of a stimulant medication resulting in increased risk 1,416 Lithium monitoring: serum levels, renal function, and 660 thyroid function Atypical antipsychotic monitoring: blood glucose levels 3,675 and lipid panel Monitoring for potential toxicities of valproic acid products TBD and/or carbamazepine Setting & Population: All patients with a diagnosis of bipolar disorder in the past two years and have pharmacy claims in the past 90 days. -

Psychiatric Complications of Treatment with Corticosteroids: Review With
Psychiatry and Clinical Neurosciences 2011; 65: 549–560 doi:10.1111/j.1440-1819.2011.02260.x Review Article Psychiatric complications of treatment with corticosteroids: Review with case reportpcn_2260 549..560 Heather A. Kenna,* MA, Amy W. Poon, MD, C. Paula de los Angeles, BA and Lorrin M. Koran, MD Department of Psychiatry and Behavioral Sciences, Stanford University Medical Center, Stanford, USA Corticosteroids are widely used in modern medicine symptoms and syndromes were identified. Data on but can result in troubling psychiatric side-effects. incidence, drug dose, risk factors, course of illness Physicians and other medical professionals should and treatment (when present) were tabulated. We be aware of the potential for these side-effects, conclude that the cumulative data indicate that psy- possible means of prevention, and efficacious chiatric complications of corticosteroid treatment are treatments. Herein, we review adult case report not rare and range from clinically significant anxiety data published during the past quarter-century on and insomnia, to severe mood and psychotic disor- adverse corticosteroid-induced psychiatric effects, ders, delirium and dementia. While tapering or dis- and present a case of corticosteroid-induced psy- continuation of the corticosteroid treatment may chotic depression. PubMed and PsychLit databases remedy these adverse side-effects, psychotropic medi- were searched using the terms ‘corticosteroids’, ‘ste- cations are often required because of the medical roids’, and the generic names of corticosteroid medi- necessity of the corticosteroid or the severity of the cations with terms for psychiatric symptoms or psychiatric symptom. Further studies are needed to syndromes, including psychosis, mania, hypomania, better understand the deleterious psychiatric effects depression, apathy, anxiety, panic, depersonaliza- associated with corticosteroids. -

Report Update 2
Drug Class Review Antiepileptic Drugs for indications other than Epilepsy Final Report Update 2 October 2008 Original Report Date: December 2004 Update 1 Report Date: May 2006 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Update 2 Marian Mcdonagh, PharmD. Kim Peterson, MS. Nancy Lee, PharmD. Sujata Thakurta, MPA:HA Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Original Report and Update 1 Southern California Evidence-based Practice Center RAND Paul Shekelle, MD, PhD, Director Copyright © 2008 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). The Drug Effectiveness Review Project governance group elected to proceed with another update of this report based on the information contained in the scan. Please see timeline on the DERP website for details on the date of its release. Prior versions of this report can be -

Irritability, Disruptive Mood Dysregulation Disorder, and Bipolar Disorder
Pediatric Mood Dysregulation: Irritability, Disruptive Mood Dysregulation Disorder, and Bipolar Disorder William P. French, MD March 24, 2018 Disclosure of Potential Conflicts Source Disclosure Pfizer, Shire, & Supernus Research Funding Pharmaceuticals Books, Intellectual Property none Advisor/ Consultant none Speakers’ Bureau Symposia Medicus Employee none In-kind Services (example: travel) Symposia Medicus Stock or Equity none Honorarium or expenses for this Symposia Medicus presentation or meeting Today’s agenda • Irritability • Disruptive Mood Dysregulation Disorder • Severe Mood Dysregulation • Pediatric Bipolar Disorder • Yes, its controversial and rare, but it does exist • DSM 5 diagnostic criteria • Assessment • Medication and non-medication treatments • Take home message Key Points in Today’s Presentation • Irritability and/or “mood swings” don’t equal pediatric bipolar disorder (PBD). Irritability (a potential presenting complaint in bipolar mania) is a non-specific, common symptom present in many childhood psychiatric illnesses. • Multiple factors have led to a significant increase the diagnosis of PBD, leading to inappropriate prescribing of medications (e.g., antipsychotics) with serious side effect burdens. • NIMH research has worked to clarify unique neurobiological substrates and clinical courses in youth presenting with chronic vs. episodic irritability • PBD, while a “zebra” and not a “horse” does occur in youth, especially in the context of a strong family history. • Youth presenting with acute mania, ideally, should