Report Update 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Silico Methods for Drug Repositioning and Drug-Drug Interaction Prediction
In silico Methods for Drug Repositioning and Drug-Drug Interaction Prediction Pathima Nusrath Hameed ORCID: 0000-0002-8118-9823 Submitted in total fulfilment of the requirements for the degree of Doctor of Philosophy Department of Mechanical Engineering THE UNIVERSITY OF MELBOURNE May 2018 Copyright © 2018 Pathima Nusrath Hameed All rights reserved. No part of the publication may be reproduced in any form by print, photoprint, microfilm or any other means without written permission from the author. Abstract Drug repositioning and drug-drug interaction (DDI) prediction are two fundamental ap- plications having a large impact on drug development and clinical care. Drug reposi- tioning aims to identify new uses for existing drugs. Moreover, understanding harmful DDIs is essential to enhance the effects of clinical care. Exploring both therapeutic uses and adverse effects of drugs or a pair of drugs have significant benefits in pharmacology. The use of computational methods to support drug repositioning and DDI prediction en- able improvements in the speed of drug development compared to in vivo and in vitro methods. This thesis investigates the consequences of employing a representative training sam- ple in achieving better performance for DDI classification. The Positive-Unlabeled Learn- ing method introduced in this thesis aims to employ representative positives as well as reliable negatives to train the binary classifier for inferring potential DDIs. Moreover, it explores the importance of a finer-grained similarity metric to represent the pairwise drug similarities. Drug repositioning can be approached by new indication detection. In this study, Anatomical Therapeutic Chemical (ATC) classification is used as the primary source to determine the indications/therapeutic uses of drugs for drug repositioning. -

Therapeutic Class Overview Anticonvulsants
Therapeutic Class Overview Anticonvulsants INTRODUCTION Epilepsy is a disease of the brain defined by any of the following (Fisher et al 2014): ○ At least 2 unprovoked (or reflex) seizures occurring > 24 hours apart; ○ 1 unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after 2 unprovoked seizures, occurring over the next 10 years; ○ Diagnosis of an epilepsy syndrome. Types of seizures include generalized seizures, focal (partial) seizures, and status epilepticus (Centers for Disease Control and Prevention [CDC] 2018, Epilepsy Foundation 2016). ○ Generalized seizures affect both sides of the brain and include: . Tonic-clonic (grand mal): begin with stiffening of the limbs, followed by jerking of the limbs and face . Myoclonic: characterized by rapid, brief contractions of body muscles, usually on both sides of the body at the same time . Atonic: characterized by abrupt loss of muscle tone; they are also called drop attacks or akinetic seizures and can result in injury due to falls . Absence (petit mal): characterized by brief lapses of awareness, sometimes with staring, that begin and end abruptly; they are more common in children than adults and may be accompanied by brief myoclonic jerking of the eyelids or facial muscles, a loss of muscle tone, or automatisms. ○ Focal seizures are located in just 1 area of the brain and include: . Simple: affect a small part of the brain; can affect movement, sensations, and emotion, without a loss of consciousness . Complex: affect a larger area of the brain than simple focal seizures and the patient loses awareness; episodes typically begin with a blank stare, followed by chewing movements, picking at or fumbling with clothing, mumbling, and performing repeated unorganized movements or wandering; they may also be called “temporal lobe epilepsy” or “psychomotor epilepsy” . -

(12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant Et Al
US 2010.0143507A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/014.3507 A1 Gant et al. (43) Pub. Date: Jun. 10, 2010 (54) CARBOXYLIC ACID INHIBITORS OF Publication Classification HISTONE DEACETYLASE, GABA (51) Int. Cl. TRANSAMINASE AND SODIUM CHANNEL A633/00 (2006.01) A 6LX 3/553 (2006.01) A 6LX 3/553 (2006.01) (75) Inventors: Thomas G. Gant, Carlsbad, CA A63L/352 (2006.01) (US); Sepehr Sarshar, Cardiff by A6II 3/19 (2006.01) the Sea, CA (US) C07C 53/128 (2006.01) A6IP 25/06 (2006.01) A6IP 25/08 (2006.01) Correspondence Address: A6IP 25/18 (2006.01) GLOBAL PATENT GROUP - APX (52) U.S. Cl. .................... 424/722:514/211.13: 514/221; 10411 Clayton Road, Suite 304 514/456; 514/557; 562/512 ST. LOUIS, MO 63131 (US) (57) ABSTRACT Assignee: AUSPEX The present invention relates to new carboxylic acid inhibi (73) tors of histone deacetylase, GABA transaminase, and/or PHARMACEUTICALS, INC., Sodium channel activity, pharmaceutical compositions Vista, CA (US) thereof, and methods of use thereof. (21) Appl. No.: 12/632,507 Formula I (22) Filed: Dec. 7, 2009 Related U.S. Application Data (60) Provisional application No. 61/121,024, filed on Dec. 9, 2008. US 2010/014.3507 A1 Jun. 10, 2010 CARBOXYLIC ACID INHIBITORS OF HISTONE DEACETYLASE, GABA TRANSAMNASE AND SODIUM CHANNEL 0001. This application claims the benefit of priority of Valproic acid U.S. provisional application No. 61/121,024, filed Dec. 9, 2008, the disclosure of which is hereby incorporated by ref 0004 Valproic acid is extensively metabolised via erence as if written herein in its entirety. -

Guidance on the Use of Mood Stabilizers for the Treatment of Bipolar Affective Disorder Version 2
Guidance on the use of mood stabilizers for the treatment of bipolar affective disorder Version 2 RATIFYING COMMITTEE DRUGS AND THERAPEUTICS GROUP DATE RATIFIED July 2015 REPLACES Version 1 dated July 2013 NEXT REVIEW DATE July 2017 POLICY AUTHORS Jules Haste, Lead Pharmacist, Brighton and Hove Members of the Pharmacy Team (contributors are listed overleaf) If you require this document in an alternative format, i.e. easy read, large text, audio or Braille please contact the pharmacy team on 01243 623349 Page 1 of 49 Contributors Jed Hewitt, Chief Pharmacist - Governance & Professional Practice James Atkinson, Pharmacist Team Leader Mental Health and Community Services Miguel Gomez, Lead Pharmacist, Worthing. Hilary Garforth, Lead Pharmacist, Chichester. Pauline Daw, Lead Pharmacist (CRHTs & AOT), East Sussex. Iftekhar Khan, Lead Pharmacist (S&F Service), East Sussex. Graham Brown, Lead Pharmacist CAMHS & EIS. Gus Fernandez, Specialist Pharmacist MI and MH Lisa Stanton, Specialist Pharmacist Early Intervention Services & Learning Disabilities. Nana Tomova, Specialist Pharmacist, Crawley. Page 2 of 49 Section Title Page Number Introduction and Key Points 4 1. General principles in the treatment of acute mania 6 2. General principles in the treatment of bipolar depression 8 3. General principles in long term treatment 10 4. Rapid cycling 13 5. Physical health 13 6. Treatment in special situations 6.1 Pregnancy 15 6.2 Breast-feeding 17 6.3 Older adults 19 6.4 Children and adolescents 22 6.5 Learning disabilities 29 6.6 Cardiac dysfunction 30 6.7 Renal dysfunction 34 6.8 Hepatic dysfunction 37 6.9 Epilepsy 41 7. The risk of switching to mania with antidepressants 43 8. -

Homicide and Associated Steroid Acute Psychosis: a Case Report
Hindawi Publishing Corporation Case Reports in Medicine Volume 2011, Article ID 564521, 4 pages doi:10.1155/2011/564521 Case Report Homicide and Associated Steroid Acute Psychosis: A Case Report G. Airagnes,1, 2 C. Rouge-Maillart,1, 3 J.-B. Garre,2, 3 and B. Gohier2 1 Service de M´edecine L´egale, CHU d’Angers, 49933 Angers Cedex 09, France 2 D´epartement de Psychiatrie et de Psychologie m´edicale, CHU d’Angers, 49933 Angers Cedex 09, France 3 IFR 132, Universit´e d’Angers, 49035 Angers, France Correspondence should be addressed to G. Airagnes, [email protected] Received 22 June 2011; Revised 26 August 2011; Accepted 26 September 2011 Academic Editor: Massimo Gallerani Copyright © 2011 G. Airagnes et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. We report the case of an old man treated with methylprednisolone for chronic lymphoid leukemia. After two months of treatment, he declared an acute steroid psychosis and beat his wife to death. Steroids were stopped and the psychotic symptoms subsided, but his condition declined very quickly. The clinical course was complicated by a major depressive disorder with suicidal ideas, due to the steroid stoppage, the leukemia progressed, and by a sudden onset of a fatal pulmonary embolism. This clinical case highlights the importance of early detection of steroid psychosis and proposes, should treatment not be stopped, a strategy of dose reduction combined with a mood stabilizer or antipsychotic treatment. -

Appendix D. Study Characteristics
Appendix D. Study Characteristics Table D1. Study characteristics Participant Author Study Study Characteristics Treatment Characteristics Outcomes Reported Characteristics Conclusions Alacqua et al., Recruitment dates: Enrolled: 73 Treatment duration: 3 mo Benefits: NR Adverse events 2008 96 Jan 2002 to Dec 2003 Analyzed: 73 Run-in phase: No occurred frequently Completed: 50 Run-in phase duration: NR Harms: Behavioral during first 3 months Country: Italy Study design: issues, dyskinesia, of treatment with Retrospective cohort GROUP 1 Permitted drugs: NR dystonia, atypical Condition N: 2 dermatologic AE, liver antipsychotics. category: Mixed Diagnostic criteria: Age, mean±SD (range): Prohibited drugs: NR function, hepatic conditions (ADHD, DSM-IV 15.5±0.7 volume, prolactin, ASD, Males %: 50 GROUP 1 prolactin-related AE, schizophrenia- Setting: Caucasian %: NR Drug name: Clozapine sedation, sleepness, related, tics) Outpatient/community Diagnostic breakdown Dosing variability: variable total AE, weight (n): psychosis (1), Target dose (mg/day): NR change Funding: NR Inclusion criteria: (1) schizophrenia (1) Daily dose (mg/day), mean±SD ≤18 yr, (2) received an Treatment naïve (n): all (range): 150±70.1 Newcastle-Ottawa incident treatment with Inpatients (n): NR Concurrent treatments: NR Scale: 6/8 stars atypical antipsychotics First episode psychosis or SSRIs during the (n): NR GROUP 2 study period Comorbidities: NR Drug name: Olanzapine Dosing variability: variable Exclusion criteria: GROUP 2 Target dose (mg/day): NR NR N: 24 Daily dose -

Mechanism of Action of Antidepressants and Mood Stabilizers
79 MECHANISM OF ACTION OF ANTIDEPRESSANTS AND MOOD STABILIZERS ROBERT H. LENOX ALAN FRAZER Bipolar disorder (BPD), the province of mood stabilizers, the more recent understanding that antidepressants share has long been considered a recurrent disorder. For more this property in UPD have focused research on long-term than 50 years, lithium, the prototypal mood stabilizer, has events, such as alterations in gene expression and neuroplas- been known to be effective not only in acute mania but ticity, that may play a significant role in stabilizing the clini- also in the prophylaxis of recurrent episodes of mania and cal course of an illness. In our view, behavioral improvement depression. By contrast, the preponderance of past research and stabilization stem from the acute pharmacologic effects in depression has focused on the major depressive episode of antidepressants and mood stabilizers; thus, both the acute and its acute treatment. It is only relatively recently that and longer-term pharmacologic effects of both classes of investigators have begun to address the recurrent nature of drugs are emphasized in this chapter. unipolar disorder (UPD) and the prophylactic use of long- term antidepressant treatment. Thus, it is timely that we address in a single chapter the most promising research rele- vant to the pharmacodynamics of both mood stabilizers and MOOD STABILIZERS antidepressants. As we have outlined in Fig. 79.1, it is possible to charac- The term mood stabilizer within the clinical setting is com- terize both the course and treatment of bipolar and unipolar monly used to refer to a class of drugs that treat BPD. -

Page 1 of 85 Reference ID: 3091122
HIGHLIGHTS OF PRESCRIBING INFORMATION As an adjunct to 2-5 mg 5-10 mg 15 mg These highlights do not include all the information needed to use ABILIFY antidepressants for the /day /day /day safely and effectively. See full prescribing information for ABILIFY. treatment of major ® ABILIFY (aripiprazole) Tablets depressive disorder – adults ® ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets (2.3) ® Irritability associated with 2 mg/day 5-10 mg/day 15 mg/day ABILIFY (aripiprazole) Oral Solution ® autistic disorder – pediatric ABILIFY (aripiprazole) Injection FOR INTRAMUSCULAR USE ONLY patients (2.4) Initial U.S. Approval: 2002 Agitation associated with 9.75 mg /1.3 30 mg/day schizophrenia or bipolar mL injected injected WARNINGS: INCREASED MORTALITY IN ELDERLY mania – adults (2.5) IM IM PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and • Oral formulations: Administer once daily without regard to meals (2) SUICIDALITY AND ANTIDEPRESSANT DRUGS • IM injection: Wait at least 2 hours between doses. Maximum daily dose See full prescribing information for complete boxed warning. 30 mg (2.5) • Elderly patients with dementia-related psychosis treated with ----------------------DOSAGE FORMS AND STRENGTHS--------------------- antipsychotic drugs are at an increased risk of death. ABILIFY is not approved for the treatment of patients with dementia-related • Tablets: 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, and 30 mg (3) psychosis. (5.1) • Orally Disintegrating Tablets: 10 mg and 15 mg (3) • Oral Solution: 1 mg/mL (3) • Children, adolescents, and young adults taking antidepressants for • Injection: 9.75 mg/1.3 mL single-dose vial (3) major depressive disorder (MDD) and other psychiatric disorders are at increased risk of suicidal thinking and behavior. -
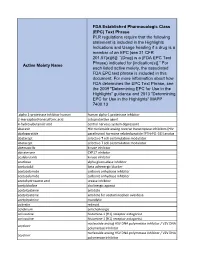
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis With
University of Rhode Island DigitalCommons@URI Pharmacy Practice Faculty Publications Pharmacy Practice 2018 Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis with Antiepileptic Drugs: An Analysis of the Food and Drug Administration Adverse Event Reporting System (FAERS) Eric P. Borrelli University of Rhode Island, [email protected] Erica Y. Lee University of Rhode Island SeFoe nelloxtw pa thige fors aaddndition addal aitutionhorsal works at: https://digitalcommons.uri.edu/php_facpubs The University of Rhode Island Faculty have made this article openly available. Please let us know how Open Access to this research benefits oy u. This is a pre-publication author manuscript of the final, published article. Terms of Use This article is made available under the terms and conditions applicable towards Open Access Policy Articles, as set forth in our Terms of Use. Citation/Publisher Attribution Borrelli EP, Lee EY, Descoteaux AM, Kogut SJ, Caffrey AR. Stevens‐Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia. 2018;00:1–7. https://doi.org/10.1111/epi.14591 Available at: https://doi.org/10.1111/epi.14591 This Article is brought to you for free and open access by the Pharmacy Practice at DigitalCommons@URI. It has been accepted for inclusion in Pharmacy Practice Faculty Publications by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. Authors Eric P. Borrelli, Erica Y. Lee, Andrew M. Descoteaux, Stephen Jon Kogut, and Aisling R. Caffrey This article is available at DigitalCommons@URI: https://digitalcommons.uri.edu/php_facpubs/124 Title: Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis with Antiepileptic Drugs: An Analysis of the Food and Drug Administration Adverse Event Reporting System (FAERS) Authors: Eric P. -

"2-Oxo-1-Pyrrolidine Derivative and Its Pharmaceutical Uses"
(19) & (11) EP 1 452 524 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 207/27 (2006.01) A61K 31/4015 (2006.01) 14.10.2009 Bulletin 2009/42 A61P 25/08 (2006.01) (21) Application number: 04007878.4 (22) Date of filing: 21.02.2001 (54) "2-oxo-1-pyrrolidine derivative and its pharmaceutical uses" "2-Oxo-1-Pyrrolidinderivate und ihre pharmazeutische Verwendung" "Dérivé de 2-oxo-1-pyrrolidine et ses applications pharmaceutique" (84) Designated Contracting States: • Talaga, Patrice AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 1170 Watermael-Boitsfort (BE) MC NL PT SE TR (74) Representative: UCB (30) Priority: 23.02.2000 GB 0004297 Intellectual Property c/o UCB S.A. (43) Date of publication of application: Intellectual Property Department 01.09.2004 Bulletin 2004/36 Allée de la Recherche 60 1070 Brussels (BE) (62) Document number(s) of the earlier application(s) in accordance with Art. 76 EPC: (56) References cited: 01925354.1 / 1 265 862 WO-A-99/13911 GB-A- 1 309 692 (73) Proprietor: UCB Pharma, S.A. • PROUS: "LEVETIRACETAM" DRUGS OF THE 1170 Brussels (BE) FUTURE, BARCELONA, ES, vol. 19, no. 2, 1994, pages 111-113, XP000908882 ISSN: 0377-8282 (72) Inventors: • FISCHER W ET AL: "Die Wirksamkeit von • Differding, Edmond Piracetam, Meclofenoxat und Vinpocetin in 1348 Ottignies-Louvain-La-Neuve (BE) verschiedenen Krampfmodellen bei der Maus" • Kenda, Benoît PHARMAZIE, VEB VERLAG VOLK UND 5080 Emines (BE) GESUNDHEIT. BERLIN, DD, vol. 46, no. -

Mood Stabilizers
Mood Stabilizers Anticonvulsants Depakene/Stavzor (valproic acid) and Depakote/Depakote ER (divalproex sodium) Equetro, Tegretol, and Tegretol-XR (carbamazepine) Lamictal (lamotrigine) Lyrica (pregabalin) Neurontin (gabapentin) Topamax (topiramate) Trileptal (oxcarbazepine) Lithium Carbonate and Lithium Citrate Lithium carbonate Lithium Citrate Lithobid Second-Generation Antipsychotics Abilify (aripiprazole) Clozaril (clozapine) Geodon (ziprasidone) Invega (paliperidone) Risperdal, Risperdal M-Tab, and Risperdal Consta (risperidone) Seroquel (quetiapine) Zyprexa and Zyprexa Zydis (olanzapine) Simply defined, mood stabilizers are medicines used in treating mood disorders such as bipolar disorder and depression. Bipolar disorder is characterized by mood swings in which the individual cycles between mania and depression. The symptoms fluctuate from euphoria and limitless energy in the manic phase to the depths of depression with little energy, guilt, sadness, decreased concentration, lack of appetite, and sleep distur- bance. Because of the wide range of symptoms, from mania to depression, bipolar disorder is often called manic depression. Mood stabilizers are used to treat acute mania, hypomania (a mild form of mania), mixed episodes (when mania and depression coexist in an episode), and depression. Following the acute episode, mood stabilizers Page 2 of 3 MOOD STABILIZERS are used in maintenance therapy to prevent the cyclical relapse of abnormal mood elevations and depressions. The goals of maintenance treatment with a mood stabilizer are to 1) reduce residual symptoms, 2) prevent manic or depressive relapse, 3) reduce the frequency of cycling into the next manic or depressive episode, 4) improve functioning, and 5) reduce the risk of suicide. Lithium Lithium was one of the first mood stabilizers used in the treatment of bipolar disorder.