Drugs Affecting the Central Nervous System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PPE Requirements Hazardous Drug Handling
This document’s purpose is only to provide general guidance. It is not a definitive interpretation for how to comply with DOSH requirements. Consult the actual NIOSH hazardous drugs list and program regulations in entirety to understand all specific compliance requirements. Minimum PPE Required Minimum PPE Required Universal (Green) - handling and disposed of using normal precautions. PPE Requirements High (Red) - double gloves, gown, eye and face protection in Low (Yellow) - handle at all times with gloves and appropriate engineering Hazardous Drug Handling addition to any necessary controls. engineering controls. Moderate (Orange) -handle at all times with gloves, gown, eye and face protection (with splash potential) and appropirate engineering controls. Tablet Open Capsule Handling only - Contained Crush/Split No alteration Crush/Split Dispensed/Common Drug Name Other Drug Name Additional Information (Formulation) and (NIOSH CATEGORY #) Minimum PPE Minimum PPE Minimum PPE Minimum PPE Required required required required abacavir (susp) (2) ziagen/epzicom/trizivir Low abacavir (tablet) (2) ziagen/epzicom/trizivir Universal Low Moderate acitretin (capsule) (3) soriatane Universal Moderate anastrazole (tablet) (1) arimidex Low Moderate High android (capsule) (3) methyltestosterone Universal Moderate apomorphine (inj sq) (2) apomorphine Moderate arthotec/cytotec (tablet) (3) diclofenac/misoprostol Universal Low Moderate astagraf XL (capsule) (2) tacrolimus Universal do not open avordart (capsule) (3) dutasteride Universal Moderate azathioprine -
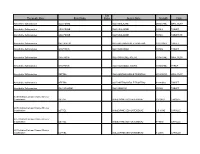
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

With LAMOTRIGINE IN
were independent of seizure type. Cardiac abnormalities occurring in epilepsy with GTCS may potentially facilitate sudden cardiac death (SUDEP). ETHOSUXIMIDE, VALPROIC ACID, AND LAMOTRIGINE IN CHILDHOOD ABSENCE EPILEPSY Efficacy, tolerability, and neuropsychological effects of ethosuximide, valproic acid, and lamotrigine in children with newly diagnosed childhood absence epilepsy were compared in a double-blind, randomized, controlled clinical trial performed at six centers in the US and organized as a Study Group. Drug doses were incrementally increased until freedom from seizures or highest tolerable dose was reached, Primary outcome was freedom from treatment failure after 16 weeks therapy, and secondary outcome was attentional dysfunction. In a total of 453 children, the freedom-from-failure rates after 16 weeks were similar for ethosuximide (53%) and valproic acid (58%), and higher than the rate for lamotrigine (29%) (P<0.001). Attentional dysfunction was more common with valproic acid (49%) than with ethosuximide (33%) (P=0.03). (Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med March 4 2010;362:790-799). (Reprints: Dr Tracy A Glauser, Cincinnati Children's Hospital, 3333 Burnett Ave, MLC 2015, Cincinnati, OH 45229. E- mail: [email protected]). COMMENT. "Older is better," is the conclusion of Vining EPG, in an editorial (N Engl J Med 2010;362:843-845). As generally accepted in US practice and confirmed by the above controlled trial, ethosuximide from the 1950s is the optimal initial therapy for childhood absence epilepsy without GTCS. Ethosuximide is equal to valproic acid in seizure control and superior in effects on attention. -

Management of Status Epilepticus
Published online: 2019-11-21 THIEME Review Article 267 Management of Status Epilepticus Ritesh Lamsal1 Navindra R. Bista1 1Department of Anaesthesiology, Tribhuvan University Teaching Address for correspondence Ritesh Lamsal, MD, DM, Department Hospital, Institute of Medicine, Tribhuvan University, Nepal of Anaesthesiology, Tribhuvan University Teaching Hospital, Institute of Medicine, Tribhuvan University,Kathmandu, Nepal (e-mail: [email protected]). J Neuroanaesthesiol Crit Care 2019;6:267–274 Abstract Status epilepticus (SE) is a life-threatening neurologic condition that requires imme- diate assessment and intervention. Over the past few decades, the duration of seizure Keywords required to define status epilepticus has shortened, reflecting the need to start thera- ► convulsive status py without the slightest delay. The focus of this review is on the management of con- epilepticus vulsive and nonconvulsive status epilepticus in critically ill patients. Initial treatment ► neurocritical care of both forms of status epilepticus includes immediate assessment and stabilization, ► nonconvulsive status and administration of rapidly acting benzodiazepine therapy followed by nonbenzodi- epilepticus azepine antiepileptic drug. Refractory and super-refractory status epilepticus (RSE and ► refractory status SRSE) pose a lot of therapeutic problems, necessitating the administration of contin- epilepticus uous infusion of high doses of anesthetic agents, and carry a high risk of debilitating ► status epilepticus morbidity as well as mortality. ► super-refractory sta- tus epilepticus Introduction occur after 30 minutes of seizure activity. However, this working definition did not indicate the need to immediately Status epilepticus (SE) is a medical and neurologic emergency commence treatment and that permanent neuronal injury that requires immediate evaluation and treatment. It is associat- could occur by the time a clinical diagnosis of SE was made. -

STATUS EPILEPTICUS in ADULTS (Convulsive Seizures in Patients Aged > 16 Years Old) Link Consultant: Dr Hannah Cock
STATUS EPILEPTICUS IN ADULTS (Convulsive Seizures in patients aged > 16 years old) Link consultant: Dr Hannah Cock Status epilepticus (SE) is defined as continuous seizure activity which has failed to self- terminate leading to a risk of neurological damage. The risks are highest with generalised tonic/clonic (convulsive) seizures. Convulsive SE may present as either a run of discreet generalised tonic/clonic seizures without full recovery in between (ie without regaining consciousness), or continuous generalised tonic/clonic seizure activity. Most convulsive seizures terminate spontaneously within 3 minutes, and do NOT need emergency treatment. Convulsive seizures lasting longer than 5 minutes, or recurring without recovery should be managed as Convulsive SE, unless the patient is known to habitually have longer seizures with self-termination (eg information from relatives, friends, or the patient’s epilepsy card or diary). The mortality and morbidity of generalised status epilepticus is high, and it is important to control fits as soon as possible, to use adequate doses of 1st and 2nd line agents, but not to over-treat patients in whom seizures have terminated but are slow to recover. GENERAL MANAGEMENT 1st stage (0-10mins). Protect the patient e.g. padded bed rails. Do not restrain. Administer oxygen. During an inter-ictal period insert an airway and then administer oxygen. Do not attempt to insert anything in the patient’s mouth during a seizure, even if the tongue is injured. Place the patient in a semi-prone position with the head down to prevent aspiration. Establish iv access. Note the time. 2nd Stage (0-30mins). Institute regular monitoring (temperature, cardiac, respiration, BP). -

K+ Channel Modulators Product ID Product Name Description D3209 Diclofenac Sodium Salt NSAID; COX-1/2 Inhibitor, Potential K+ Channel Modulator
K+ Channel Modulators Product ID Product Name Description D3209 Diclofenac Sodium Salt NSAID; COX-1/2 inhibitor, potential K+ channel modulator. G4597 18β-Glycyrrhetinic Acid Triterpene glycoside found in Glycyrrhiza; 15-HPGDH inhibitor, hERG and KCNA3/Kv1.3 K+ channel blocker. A4440 Allicin Organosulfur found in garlic, binds DNA; inwardly rectifying K+ channel activator, L-type Ca2+ channel blocker. P6852 Propafenone Hydrochloride β-adrenergic antagonist, Kv1.4 and K2P2 K+ channel blocker. P2817 Phentolamine Hydrochloride ATP-sensitive K+ channel activator, α-adrenergic antagonist. P2818 Phentolamine Methanesulfonate ATP-sensitive K+ channel activator, α-adrenergic antagonist. T7056 Troglitazone Thiazolidinedione; PPARγ agonist, ATP-sensitive K+ channel blocker. G3556 Ginsenoside Rg3 Triterpene saponin found in species of Panax; γ2 GABA-A agonist, Kv7.1 K+ channel activator, α10 nAChR antagonist. P6958 Protopanaxatriol Triterpene sapogenin found in species of Panax; GABA-A/C antagonist, slow-activating delayed rectifier K+ channel blocker. V3355 Vindoline Semi-synthetic vinca alkaloid found in Catharanthus; Kv2.1 K+ channel blocker and H+/K+ ATPase inhibitor. A5037 Amiodarone Hydrochloride Voltage-gated Na+, Ca2+, K+ channel blocker, α/β-adrenergic antagonist, FIASMA. B8262 Bupivacaine Hydrochloride Monohydrate Amino amide; voltage-gated Na+, BK/SK, Kv1, Kv3, TASK-2 K+ channel inhibitor. C0270 Carbamazepine GABA potentiator, voltage-gated Na+ and ATP-sensitive K+ channel blocker. C9711 Cyclovirobuxine D Found in Buxus; hERG K+ channel inhibitor. D5649 Domperidone D2/3 antagonist, hERG K+ channel blocker. G4535 Glimepiride Sulfonylurea; ATP-sensitive K+ channel blocker. G4634 Glipizide Sulfonylurea; ATP-sensitive K+ channel blocker. I5034 Imiquimod Imidazoquinoline nucleoside analog; TLR-7/8 agonist, KCNA1/Kv1.1 and KCNA2/Kv1.2 K+ channel partial agonist, TREK-1/ K2P2 and TRAAK/K2P4 K+ channel blocker. -

In Silico Methods for Drug Repositioning and Drug-Drug Interaction Prediction
In silico Methods for Drug Repositioning and Drug-Drug Interaction Prediction Pathima Nusrath Hameed ORCID: 0000-0002-8118-9823 Submitted in total fulfilment of the requirements for the degree of Doctor of Philosophy Department of Mechanical Engineering THE UNIVERSITY OF MELBOURNE May 2018 Copyright © 2018 Pathima Nusrath Hameed All rights reserved. No part of the publication may be reproduced in any form by print, photoprint, microfilm or any other means without written permission from the author. Abstract Drug repositioning and drug-drug interaction (DDI) prediction are two fundamental ap- plications having a large impact on drug development and clinical care. Drug reposi- tioning aims to identify new uses for existing drugs. Moreover, understanding harmful DDIs is essential to enhance the effects of clinical care. Exploring both therapeutic uses and adverse effects of drugs or a pair of drugs have significant benefits in pharmacology. The use of computational methods to support drug repositioning and DDI prediction en- able improvements in the speed of drug development compared to in vivo and in vitro methods. This thesis investigates the consequences of employing a representative training sam- ple in achieving better performance for DDI classification. The Positive-Unlabeled Learn- ing method introduced in this thesis aims to employ representative positives as well as reliable negatives to train the binary classifier for inferring potential DDIs. Moreover, it explores the importance of a finer-grained similarity metric to represent the pairwise drug similarities. Drug repositioning can be approached by new indication detection. In this study, Anatomical Therapeutic Chemical (ATC) classification is used as the primary source to determine the indications/therapeutic uses of drugs for drug repositioning. -

Therapeutic Potential of RQ-00311651, a Novel T-Type Ca
Research Paper Therapeutic potential of RQ-00311651, a novel T-type Ca21 channel blocker, in distinct rodent models for neuropathic and visceral pain Fumiko Sekiguchia, Yuma Kawaraa, Maho Tsubotaa, Eri Kawakamia, Tomoka Ozakia, Yudai Kawaishia, Shiori Tomitaa, Daiki Kanaokaa, Shigeru Yoshidab, Tsuyako Ohkuboc, Atsufumi Kawabataa,* Abstract 21 T-type Ca channels (T channels), particularly Cav3.2 among the 3 isoforms, play a role in neuropathic and visceral pain. We thus characterized the effects of RQ-00311651 (RQ), a novel T-channel blocker, in HEK293 cells transfected with human Cav3.1 or 21 Cav3.2 by electrophysiological and fluorescent Ca signaling assays, and also evaluated the antiallodynic/antihyperalgesic activity of RQ in somatic, visceral, and neuropathic pain models in rodents. RQ-00311651 strongly suppressed T currents when tested at holding potentials of 265 ; 260 mV, but not 280 mV, in the Cav3.1- or Cav3.2-expressing cells. RQ-00311651 also inhibited high K1-induced Ca21 signaling in those cells. In mice, RQ, administered intraperitoneally (i.p.) at 5 to 20 mg/kg or orally at 20 to 40 mg/kg, significantly suppressed the somatic hyperalgesia and visceral pain-like nociceptive behavior/referred hyperalgesia caused by intraplantar and intracolonic administration of NaHS or Na2S, H2S donors, respectively, which involve the enhanced activity of Cav3.2 channels. RQ-00311651, given i.p. at 5 to 20 mg/kg, exhibited antiallodynic or antihyperalgesic activity in rats with spinal nerve injury–induced neuropathy or in rats and mice with paclitaxel-induced neuropathy. Oral and i.p. RQ at 10 to 20 mg/kg also suppressed the visceral nociceptive behavior and/or referred hyperalgesia accompanying cerulein-induced acute pancreatitis and cyclophosphamide-induced cystitis in mice. -

Z944: an Oral T-Type Calcium Channel Modulator for the Treatment of Pain Margaret S
Z944: An oral T-type calcium channel modulator for the treatment of pain Margaret S. Lee, PhD Ion Channel Retreat 2014, June 25, 2014 T-type Calcium Channels: A Novel Target for Pain and Other CNS Disorders • T-type calcium channels are voltage gated and comprised of three subtypes: Cav3.1, Cav3.2 & Cav3.3 • Expressed in Central and Peripheral Nervous System, including primary afferent, dorsal horn neurons, thalamus and somatosensory cortex • Contribute to neuronal excitability, synaptic excitation, burst firing and action potential trains, and also lower threshold for action potentials Pain Signaling Thalamocortical Connectivity Source: Adapted from Zamponi, et al., Brain Res. Reviews. 2009 Source: Adapted from Park, et al., Frontiers Neural Circuits. 2013 • Rodent neuropathic and IBS pain models exhibit increased • Thalamocortical dysrythmia linked to CNS indications, e.g. T-type current density motor, neuropsychiatric and chronic pain syndromes • Gene knockout or antisense reduces pain in neuropathic, • Mutations in T-type channels are found in rodent and acute and visceral pain models human excitability disorders • T-type channel blockers attenuate neuropathic, • Approved anti-convulsants (e.g. ethosuximide, valproate) inflammatory, acute and visceral pain in animal models target T-type channels © Copyright Neuromed 2 Z944 is a Potent, Selective Blocker of T-type Calcium Channels IC50 (nM) Channel 30% Fold-Selectivity Closed Inactivated (30% Inactivated) CaV3.1(human, exogenous) 50 130 1 CaV3.2 (human, exogenous) 160 540 3.2 CaV3.3 (human, exogenous) 110 260 2.2 N-type (rat, exogenous) 11,000 150,000 220 L-type cardiac calcium (rat CaV1.2) 32,000 -- 640 Cardiac Sodium (human NaV1.5) 100,000 -- 2000 hERG channel (human) 7,800 -- 156 • Displays enhanced potency for the inactivated state across T-type channels • Z944 block of Cav3.2 is more pronounced during high-frequency firing • Z944 has >150-fold selectivity vs. -

Gabitril® (Tiagabine)
Gabitril (Tiagabine) What is Gabitril (Tiagabine)? Gabitril is an anti-epileptic drug that has been used to treat patients with epilepsy since 1997. It is helpful in the treatment of partial seizures. Starting the Medicine: We usually gradually increase the dose, until your body gets adjusted to the medication. Since each patient is unique in that he/she breaks down the medication differently or may need a higher or lower dosage to control their seizures, there is no standard dose that is appropriate for all patients. What dosages does the pill come in and what does it look like? 4 mg round, yellow tablet 12 mg oval, green tablet 16 mg oval, blue tablet 20 mg oval, pink tablet What Side Effects Can Be Caused By Gabitril? Side effects can be dose related (common) or idiosyncratic (rare): Common Dose - Related Side Effects: Dizziness, drowsiness, difficulty concentrating, or nausea may occur. If you have these side effects, your doctor may: • spread out the dose more frequently during the day • slow the rate of dose increase Comprehensive Epilepsy Center (734) 936-9020 - 1 - • instruct you to take the pills with food since this will slow the rate at which the medicine gets into the blood, but will not affect the total amount that is absorbed. Rare Side Effects: Rare side effects may include: difficulty sleeping, nervousness, or balance problems. Skin Rash: An allergic reaction, or rash, may occur with Gabitril. If this occurs, notify your doctor immediately. Pregnancy: Currently, no information is available about the effects of Gabitril during pregnancy. Any woman taking Gabitril should discuss this issue with her doctor BEFORE becoming pregnant. -

Therapeutic Class Overview Anticonvulsants
Therapeutic Class Overview Anticonvulsants INTRODUCTION Epilepsy is a disease of the brain defined by any of the following (Fisher et al 2014): ○ At least 2 unprovoked (or reflex) seizures occurring > 24 hours apart; ○ 1 unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after 2 unprovoked seizures, occurring over the next 10 years; ○ Diagnosis of an epilepsy syndrome. Types of seizures include generalized seizures, focal (partial) seizures, and status epilepticus (Centers for Disease Control and Prevention [CDC] 2018, Epilepsy Foundation 2016). ○ Generalized seizures affect both sides of the brain and include: . Tonic-clonic (grand mal): begin with stiffening of the limbs, followed by jerking of the limbs and face . Myoclonic: characterized by rapid, brief contractions of body muscles, usually on both sides of the body at the same time . Atonic: characterized by abrupt loss of muscle tone; they are also called drop attacks or akinetic seizures and can result in injury due to falls . Absence (petit mal): characterized by brief lapses of awareness, sometimes with staring, that begin and end abruptly; they are more common in children than adults and may be accompanied by brief myoclonic jerking of the eyelids or facial muscles, a loss of muscle tone, or automatisms. ○ Focal seizures are located in just 1 area of the brain and include: . Simple: affect a small part of the brain; can affect movement, sensations, and emotion, without a loss of consciousness . Complex: affect a larger area of the brain than simple focal seizures and the patient loses awareness; episodes typically begin with a blank stare, followed by chewing movements, picking at or fumbling with clothing, mumbling, and performing repeated unorganized movements or wandering; they may also be called “temporal lobe epilepsy” or “psychomotor epilepsy” . -

CEREBYX® (Fosphenytoin Sodium Injection)
NDA 020450 Cerebyx (fosphenytoin sodium) Injection FDA Approved Labeling Text dated 10/2011 Page 1 of 22 CEREBYX® (Fosphenytoin Sodium Injection) WARNING: CARDIOVASCULAR RISK ASSOCIATED WITH RAPID INFUSION RATES The rate of intravenous CEREBYX administration should not exceed 150 mg phenytoin sodium equivalents (PE) per minute because of the risk of severe hypotension and cardiac arrhythmias. Careful cardiac monitoring is needed during and after administering intravenous CEREBYX. Although the risk of cardiovascular toxicity increases with infusion rates above the recommended infusion rate, these events have also been reported at or below the recommended infusion rate. Reduction in rate of administration or discontinuation of dosing may be needed (see WARNINGS and DOSAGE AND ADMINISTRATION). DESCRIPTION CEREBYX® (fosphenytoin sodium injection) is a prodrug intended for parenteral administration; its active metabolite is phenytoin. 1.5 mg of fosphenytoin sodium is equivalent to 1 mg phenytoin sodium, and is referred to as 1 mg phenytoin sodium equivalents (PE). The amount and concentration of fosphenytoin is always expressed in terms of mg PE. CEREBYX is marketed in 2 mL vials containing a total of 100 mg PE and 10 mL vials containing a total of 500 mg PE. The concentration of each vial is 50 mg PE/mL. CEREBYX is supplied in vials as a ready-mixed solution in Water for Injection, USP, and Tromethamine, USP (TRIS), buffer adjusted to pH 8.6 to 9.0 with either Hydrochloric Acid, NF, or Sodium Hydroxide, NF. CEREBYX is a clear, colorless to pale yellow, sterile solution. The chemical name of fosphenytoin is 5,5-diphenyl-3-[(phosphonooxy)methyl]-2,4 imidazolidinedione disodium salt.