A Unique Collection of Neurodevelopmental Genes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Gene Expression Data for Gene Ontology
ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Master of Science Robert Daniel Macholan May 2011 ANALYSIS OF GENE EXPRESSION DATA FOR GENE ONTOLOGY BASED PROTEIN FUNCTION PREDICTION Robert Daniel Macholan Thesis Approved: Accepted: _______________________________ _______________________________ Advisor Department Chair Dr. Zhong-Hui Duan Dr. Chien-Chung Chan _______________________________ _______________________________ Committee Member Dean of the College Dr. Chien-Chung Chan Dr. Chand K. Midha _______________________________ _______________________________ Committee Member Dean of the Graduate School Dr. Yingcai Xiao Dr. George R. Newkome _______________________________ Date ii ABSTRACT A tremendous increase in genomic data has encouraged biologists to turn to bioinformatics in order to assist in its interpretation and processing. One of the present challenges that need to be overcome in order to understand this data more completely is the development of a reliable method to accurately predict the function of a protein from its genomic information. This study focuses on developing an effective algorithm for protein function prediction. The algorithm is based on proteins that have similar expression patterns. The similarity of the expression data is determined using a novel measure, the slope matrix. The slope matrix introduces a normalized method for the comparison of expression levels throughout a proteome. The algorithm is tested using real microarray gene expression data. Their functions are characterized using gene ontology annotations. The results of the case study indicate the protein function prediction algorithm developed is comparable to the prediction algorithms that are based on the annotations of homologous proteins. -

CCT2 Antibody A
Revision 5 C 0 2 - t CCT2 Antibody a e r o t S Orders: 877-616-CELL (2355) [email protected] Support: 877-678-TECH (8324) 1 6 Web: [email protected] 5 www.cellsignal.com 3 # 3 Trask Lane Danvers Massachusetts 01923 USA For Research Use Only. Not For Use In Diagnostic Procedures. Applications: Reactivity: Sensitivity: MW (kDa): Source: UniProt ID: Entrez-Gene Id: WB, IP H M R Mk Endogenous 54 Rabbit P78371 10576 Product Usage Information 6. McCormack, E.A. et al. (2001) J Struct Biol 135, 198-204. Application Dilution Western Blotting 1:1000 Immunoprecipitation 1:100 Storage Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA and 50% glycerol. Store at –20°C. Do not aliquot the antibody. Specificity / Sensitivity CCT2 Antibody detects endogenous levels of total CCT2 protein. Species Reactivity: Human, Mouse, Rat, Monkey Source / Purification Polyclonal antibodies are produced by immunizing animals with a synthetic peptide corresponding to human CCT2. Antibodies are purified by protein A and peptide affinity chromatography. Background CCT2 is one of eight largely unrelated subunit proteins found in a protein chaperone complex known as the chaperonin-containing TCP-1 (CCT) or TRiC complex. The CCT complex is an abundanct cytoslic component that is credited with helping newly synthesized polypeptides adopt the correct conformation (1). Proteins that fold and assemble with the help of CCT include the cytoskeletal proteins actin and tubulin as well as up to 15% of newly synthesized eukaryotic proteins (2). CCT2 is the β-subunit of the chaperone complex and is one of several CCT proteins that exhibit increased expression in response to stress. -
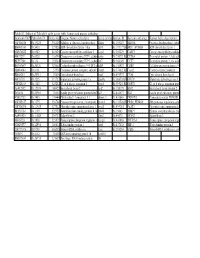
Table S3: Subset of Zebrafish Early Genes with Human And
Table S3: Subset of Zebrafish early genes with human and mouse orthologs Genbank ID(ZFZebrafish ID Entrez GenUnigene Name (zebrafish) Gene symbo Human ID Humann ortholog Human Gene description AW116838 Dr.19225 336425 Aldolase a, fructose-bisphosphate aldoa Hs.155247 ALDOA Fructose-bisphosphate aldola BM005100 Dr.5438 327026 ADP-ribosylation factor 1 like arf1l Hs.119177||HsARF1_HUMAN ADP-ribosylation factor 1 AW076882 Dr.6582 403025 Cancer susceptibility candidate 3 casc3 Hs.350229 CASC3 Cancer susceptibility candidat AI437239 Dr.6928 116994 Chaperonin containing TCP1, subun cct6a Hs.73072||Hs.CCT6A T-complex protein 1, zeta sub BE557308 Dr.134 192324 Chaperonin containing TCP1, subun cct7 Hs.368149 CCT7 T-complex protein 1, eta subu BG303647 Dr.26326 321602 Cyclin-dependent kinase 9 (CDC2-recdk9 Hs.150423 CDK9 Cell division protein kinase 9 AB040044 Dr.8161 57970 Coatomer protein complex, subunit zcopz1 Hs.37482||Hs.Copz2 Coatomer zeta-2 subunit BI888253 Dr.20911 30436 Eyes absent homolog 1 eya1 Hs.491997 EYA4 Eyes absent homolog 4 AI878758 Dr.3225 317737 Glutamate dehydrogenase 1a glud1a Hs.368538||HsGLUD1 Glutamate dehydrogenase 1, AW128619 Dr.1388 325284 G1 to S phase transition 1 gspt1 Hs.59523||Hs.GSPT1 G1 to S phase transition prote AF412832 Dr.12595 140427 Heat shock factor 2 hsf2 Hs.158195 HSF2 Heat shock factor protein 2 D38454 Dr.20916 30151 Insulin gene enhancer protein Islet3 isl3 Hs.444677 ISL2 Insulin gene enhancer protein AY052752 Dr.7485 170444 Pbx/knotted 1 homeobox 1.1 pknox1.1 Hs.431043 PKNOX1 Homeobox protein PKNOX1 -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

CCT6A Sirna (Mouse)
For research purposes only, not for human use Product Data Sheet CCT6A siRNA (Mouse) Catalog # Source Reactivity Applications CRM0566 Synthetic M RNAi Description siRNA to inhibit CCT6A expression using RNA interference Specificity CCT6A siRNA (Mouse) is a target-specific 19-23 nt siRNA oligo duplexes designed to knock down gene expression. Form Lyophilized powder Gene Symbol CCT6A Alternative Names CCT6; CCTZ; CCTZ1; T-complex protein 1 subunit zeta; TCP-1-zeta; CCT-zeta-1 Entrez Gene 12466 (Mouse) SwissProt P80317 (Mouse) Purity > 97% Quality Control Oligonucleotide synthesis is monitored base by base through trityl analysis to ensure appropriate coupling efficiency. The oligo is subsequently purified by affinity-solid phase extraction. The annealed RNA duplex is further analyzed by mass spectrometry to verify the exact composition of the duplex. Each lot is compared to the previous lot by mass spectrometry to ensure maximum lot-to-lot consistency. Components We offers pre-designed sets of 3 different target-specific siRNA oligo duplexes of mouse CCT6A gene. Each vial contains 5 nmol of lyophilized siRNA. The duplexes can be transfected individually or pooled together to achieve knockdown of the target gene, which is most commonly assessed by qPCR or western blot. Our siRNA oligos are also chemically modified (2’-OMe) at no extra charge for increased stability and enhanced knockdown in vitro and in vivo. Directions for Use We recommends transfection with 100 nM siRNA 48 to 72 hours prior to cell lysis. Application key: E- ELISA, WB- -

Overexpressing CCT6A Contributes to Cancer Cell Growth by Affecting the G1-To-S Phase Transition and Predicts a Negative Prognosis in Hepatocellular Carcinoma
OncoTargets and Therapy Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Overexpressing CCT6A Contributes To Cancer Cell Growth By Affecting The G1-To-S Phase Transition And Predicts A Negative Prognosis In Hepatocellular Carcinoma This article was published in the following Dove Press journal: OncoTargets and Therapy Guofen Zeng1,* Purpose: To determine the oncogenic role of the sixth subunit of chaperonin-containing Jialiang Wang2,* tailless complex polypeptide 1 (CCT6A) in hepatocellular carcinoma (HCC) and address the Yanlin Huang 1,* correlation of CCT6A with clinicopathological characteristics and survival. Additionally, this Yifan Lian 2 study aimed to explore the effect of CCT6A on HCC cells and the underlying mechanisms. Dongmei Chen2 Methods: We searched for levels of CCT6A expression in the Oncomine database and GEPIA database, which was then validated by analyzing cancer and adjacent non-cancerous Huan Wei 2 tissues of HCC patients using quantitative PCR, Western blot, and immunohistochemistry Chaoshuang Lin1 1,2 assays. The relationship between CCT6A expression and survival was analyzed from the Yuehua Huang GEPIA database and confirmed by immunohistochemistry assays of 133 HCC tissue sec- 1Department of Infectious Diseases, The tions. In addition, the effect of depleting CCT6A on cell proliferation was assessed by CCK- fi Third Af liated Hospital of Sun Yat-sen 8 and colony formation assays. Cell cycle analysis, immunofluorescence assays, GSEA University, Guangzhou 510630, Guangdong, People’s Republic of China; analysis, and cyclin D expression analyzed by Western blot were used to explore the possible 2Guangdong Provincial Key Laboratory of underlying mechanism how dysregulated CCT6A affect the proliferation of HCC. -

Supplementary Figures and Tables
SUPPLEMENTARY DATA Supplementary Figure 1. Isolation and culture of endothelial cells from surgical specimens of FVM. (A) Representative pre-surgical fundus photograph of a right eye exhibiting a FVM encroaching on the optic nerve (dashed line) causing tractional retinal detachment with blot hemorrhages throughout retina (arrow heads). (B) Magnetic beads (arrows) allow for separation and culturing of enriched cell populations from surgical specimens (scale bar = 100 μm). (C) Cultures of isolated cells stained positively for CD31 representing a successfully isolated enriched population (scale bar = 40 μm). ©2017 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1035/-/DC1 SUPPLEMENTARY DATA Supplementary Figure 2. Efficient siRNA knockdown of RUNX1 expression and function demonstrated by qRT-PCR, Western Blot, and scratch assay. (A) RUNX1 siRNA induced a 60% reduction of RUNX1 expression measured by qRT-PCR 48 hrs post-transfection whereas expression of RUNX2 and RUNX3, the two other mammalian RUNX orthologues, showed no significant changes, indicating specificity of our siRNA. Functional inhibition of Runx1 signaling was demonstrated by a 330% increase in insulin-like growth factor binding protein-3 (IGFBP3) RNA expression level, a known target of RUNX1 inhibition. Western blot demonstrated similar reduction in protein levels. (B) siRNA- 2’s effect on RUNX1 was validated by qRT-PCR and western blot, demonstrating a similar reduction in both RNA and protein. Scratch assay demonstrates functional inhibition of RUNX1 by siRNA-2. ns: not significant, * p < 0.05, *** p < 0.001 ©2017 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1035/-/DC1 SUPPLEMENTARY DATA Supplementary Table 1. -

Open Full Page
Published OnlineFirst February 10, 2017; DOI: 10.1158/2159-8290.CD-16-1045 RESEARCH ARTICLE Interaction Landscape of Inherited Polymorphisms with Somatic Events in Cancer Hannah Carter 1 , 2 , 3 , 4 , Rachel Marty 5 , Matan Hofree 6 , Andrew M. Gross 5 , James Jensen 5 , Kathleen M. Fisch1,2,3,7 , Xingyu Wu 2 , Christopher DeBoever 5 , Eric L. Van Nostrand 4,8 , Yan Song 4,8 , Emily Wheeler 4,8 , Jason F. Kreisberg 1,3 , Scott M. Lippman 2 , Gene W. Yeo 4,8 , J. Silvio Gutkind 2 , 3 , and Trey Ideker 1 , 2 , 3 , 4 , 5,6 Downloaded from cancerdiscovery.aacrjournals.org on September 27, 2021. © 2017 American Association for Cancer Research. Published OnlineFirst February 10, 2017; DOI: 10.1158/2159-8290.CD-16-1045 ABSTRACT Recent studies have characterized the extensive somatic alterations that arise dur- ing cancer. However, the somatic evolution of a tumor may be signifi cantly affected by inherited polymorphisms carried in the germline. Here, we analyze genomic data for 5,954 tumors to reveal and systematically validate 412 genetic interactions between germline polymorphisms and major somatic events, including tumor formation in specifi c tissues and alteration of specifi c cancer genes. Among germline–somatic interactions, we found germline variants in RBFOX1 that increased incidence of SF3B1 somatic mutation by 8-fold via functional alterations in RNA splicing. Similarly, 19p13.3 variants were associated with a 4-fold increased likelihood of somatic mutations in PTEN. In support of this associ- ation, we found that PTEN knockdown sensitizes the MTOR pathway to high expression of the 19p13.3 gene GNA11 . -

Role and Regulation of the P53-Homolog P73 in the Transformation of Normal Human Fibroblasts
Role and regulation of the p53-homolog p73 in the transformation of normal human fibroblasts Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Bayerischen Julius-Maximilians-Universität Würzburg vorgelegt von Lars Hofmann aus Aschaffenburg Würzburg 2007 Eingereicht am Mitglieder der Promotionskommission: Vorsitzender: Prof. Dr. Dr. Martin J. Müller Gutachter: Prof. Dr. Michael P. Schön Gutachter : Prof. Dr. Georg Krohne Tag des Promotionskolloquiums: Doktorurkunde ausgehändigt am Erklärung Hiermit erkläre ich, dass ich die vorliegende Arbeit selbständig angefertigt und keine anderen als die angegebenen Hilfsmittel und Quellen verwendet habe. Diese Arbeit wurde weder in gleicher noch in ähnlicher Form in einem anderen Prüfungsverfahren vorgelegt. Ich habe früher, außer den mit dem Zulassungsgesuch urkundlichen Graden, keine weiteren akademischen Grade erworben und zu erwerben gesucht. Würzburg, Lars Hofmann Content SUMMARY ................................................................................................................ IV ZUSAMMENFASSUNG ............................................................................................. V 1. INTRODUCTION ................................................................................................. 1 1.1. Molecular basics of cancer .......................................................................................... 1 1.2. Early research on tumorigenesis ................................................................................. 3 1.3. Developing -

Maser: One-Stop Platform for NGS Big Data from Analysis to Visualization
Database, 2018, 1–12 doi: 10.1093/database/bay027 Database tool Database tool Maser: one-stop platform for NGS big data from analysis to visualization Sonoko Kinjo1, Norikazu Monma2, Sadahiko Misu3, Norikazu Kitamura1, Junichi Imoto1, Kazutoshi Yoshitake4, Takashi Gojobori5 and Kazuho Ikeo1,6,* 1Center for Information Biology, National Institute of Genetics, 1111 Yata, Mishima, Shizuoka 411-8540, Japan, 2Hitachi, Ltd, Tokyo, Japan, 3BITS Co., Ltd, Tokyo, Japan, 4Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan, 5Computational Bioscience Research Center, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia and 6Department of Genetics, SOKENDAI, Mishima, Japan *Corresponding author: Tel: þ81 55 981 6851; Fax: þ81 55 981 6848; E-mail: [email protected] Citation details: Kinjo,S., Monma,N., Misu,S. et al. Maser: one-stop platform for NGS big data from analysis to visualiza- tion. Database (2018) Vol. 2018: article ID bay027; doi:10.1093/database/bay027 Received 15 September 2017; Revised 22 February 2018; Accepted 26 February 2018 Abstract A major challenge in analyzing the data from high-throughput next-generation sequenc- ing (NGS) is how to handle the huge amounts of data and variety of NGS tools and visu- alize the resultant outputs. To address these issues, we developed a cloud-based data analysis platform, Maser (Management and Analysis System for Enormous Reads), and an original genome browser, Genome Explorer (GE). Maser enables users to manage up to 2 terabytes of data to conduct analyses with easy graphical user interface operations and offers analysis pipelines in which several individual tools are combined as a single pipeline for very common and standard analyses. -

The Unfolded Protein Response and Its Potential Role In
F1000Research 2016, 4:103 Last updated: 22 JUL 2020 RESEARCH ARTICLE The unfolded protein response and its potential role in Huntington's disease elucidated by a systems biology approach [version 2; peer review: 2 approved] Ravi Kiran Reddy Kalathur1, Joaquin Giner-Lamia1, Susana Machado1, Tania Barata1, Kameshwar R S Ayasolla1, Matthias E. Futschik1,2 1Centre for Biomedical Research, University of Algarve, Faro, 8005-139, Portugal 2Centre of Marine Sciences, University of Algarve, Faro, 8005-139, Portugal First published: 01 May 2015, 4:103 Open Peer Review v2 https://doi.org/10.12688/f1000research.6358.1 Latest published: 02 Mar 2016, 4:103 https://doi.org/10.12688/f1000research.6358.2 Reviewer Status Abstract Invited Reviewers Huntington ́s disease (HD) is a progressive, neurodegenerative disease 1 2 with a fatal outcome. Although the disease-causing gene (huntingtin) has been known for over 20 years, the exact mechanisms leading to neuronal version 2 cell death are still controversial. One potential mechanism contributing to (revision) report the massive loss of neurons observed in the brain of HD patients could be 02 Mar 2016 the unfolded protein response (UPR) activated by accumulation of misfolded proteins in the endoplasmic reticulum (ER). As an adaptive response to counter-balance accumulation of un- or misfolded proteins, the version 1 UPR upregulates transcription of chaperones, temporarily attenuates new 01 May 2015 report report translation, and activates protein degradation via the proteasome. However, persistent ER stress and an activated UPR can also cause apoptotic cell death. Although different studies have indicated a role for the Scott Zeitlin, University of Virginia, UPR in HD, the evidence remains inconclusive. -

CCT6A Rabbit Pab
Leader in Biomolecular Solutions for Life Science CCT6A Rabbit pAb Catalog No.: A3589 1 Publications Basic Information Background Catalog No. The protein encoded by this gene is a molecular chaperone that is a member of the A3589 chaperonin containing TCP1 complex (CCT), also known as the TCP1 ring complex (TRiC). This complex consists of two identical stacked rings, each containing eight different Observed MW proteins. Unfolded polypeptides enter the central cavity of the complex and are folded 58kDa in an ATP-dependent manner. The complex folds various proteins, including actin and tubulin. Alternate transcriptional splice variants of this gene, encoding different Calculated MW isoforms, have been characterized. In addition, several pseudogenes of this gene have 53kDa/58kDa been located. Category Primary antibody Applications WB,IHC Cross-Reactivity Human, Mouse, Rat Recommended Dilutions Immunogen Information WB 1:500 - 1:2000 Gene ID Swiss Prot 908 P40227 IHC 1:50 - 1:100 Immunogen Recombinant fusion protein containing a sequence corresponding to amino acids 80-250 of human CCT6A (NP_001753.1). Synonyms CCT6A;CCT-zeta;CCT-zeta-1;CCT6;Cctz;HTR3;MoDP-2;TCP-1-zeta;TCP20;TCPZ;TTCP20 Contact Product Information www.abclonal.com Source Isotype Purification Rabbit IgG Affinity purification Storage Store at -20℃. Avoid freeze / thaw cycles. Buffer: PBS with 0.02% sodium azide,50% glycerol,pH7.3. Validation Data Western blot analysis of extracts of various cell lines, using CCT6A antibody (A3589) at 1:1000 dilution. Secondary antibody: HRP Goat Anti-Rabbit IgG (H+L) (AS014) at 1:10000 dilution. Lysates/proteins: 25ug per lane. Blocking buffer: 3% nonfat dry milk in TBST.