CCT6A Sirna (Mouse)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
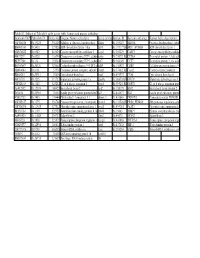
Table S3: Subset of Zebrafish Early Genes with Human And
Table S3: Subset of Zebrafish early genes with human and mouse orthologs Genbank ID(ZFZebrafish ID Entrez GenUnigene Name (zebrafish) Gene symbo Human ID Humann ortholog Human Gene description AW116838 Dr.19225 336425 Aldolase a, fructose-bisphosphate aldoa Hs.155247 ALDOA Fructose-bisphosphate aldola BM005100 Dr.5438 327026 ADP-ribosylation factor 1 like arf1l Hs.119177||HsARF1_HUMAN ADP-ribosylation factor 1 AW076882 Dr.6582 403025 Cancer susceptibility candidate 3 casc3 Hs.350229 CASC3 Cancer susceptibility candidat AI437239 Dr.6928 116994 Chaperonin containing TCP1, subun cct6a Hs.73072||Hs.CCT6A T-complex protein 1, zeta sub BE557308 Dr.134 192324 Chaperonin containing TCP1, subun cct7 Hs.368149 CCT7 T-complex protein 1, eta subu BG303647 Dr.26326 321602 Cyclin-dependent kinase 9 (CDC2-recdk9 Hs.150423 CDK9 Cell division protein kinase 9 AB040044 Dr.8161 57970 Coatomer protein complex, subunit zcopz1 Hs.37482||Hs.Copz2 Coatomer zeta-2 subunit BI888253 Dr.20911 30436 Eyes absent homolog 1 eya1 Hs.491997 EYA4 Eyes absent homolog 4 AI878758 Dr.3225 317737 Glutamate dehydrogenase 1a glud1a Hs.368538||HsGLUD1 Glutamate dehydrogenase 1, AW128619 Dr.1388 325284 G1 to S phase transition 1 gspt1 Hs.59523||Hs.GSPT1 G1 to S phase transition prote AF412832 Dr.12595 140427 Heat shock factor 2 hsf2 Hs.158195 HSF2 Heat shock factor protein 2 D38454 Dr.20916 30151 Insulin gene enhancer protein Islet3 isl3 Hs.444677 ISL2 Insulin gene enhancer protein AY052752 Dr.7485 170444 Pbx/knotted 1 homeobox 1.1 pknox1.1 Hs.431043 PKNOX1 Homeobox protein PKNOX1 -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

Overexpressing CCT6A Contributes to Cancer Cell Growth by Affecting the G1-To-S Phase Transition and Predicts a Negative Prognosis in Hepatocellular Carcinoma
OncoTargets and Therapy Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Overexpressing CCT6A Contributes To Cancer Cell Growth By Affecting The G1-To-S Phase Transition And Predicts A Negative Prognosis In Hepatocellular Carcinoma This article was published in the following Dove Press journal: OncoTargets and Therapy Guofen Zeng1,* Purpose: To determine the oncogenic role of the sixth subunit of chaperonin-containing Jialiang Wang2,* tailless complex polypeptide 1 (CCT6A) in hepatocellular carcinoma (HCC) and address the Yanlin Huang 1,* correlation of CCT6A with clinicopathological characteristics and survival. Additionally, this Yifan Lian 2 study aimed to explore the effect of CCT6A on HCC cells and the underlying mechanisms. Dongmei Chen2 Methods: We searched for levels of CCT6A expression in the Oncomine database and GEPIA database, which was then validated by analyzing cancer and adjacent non-cancerous Huan Wei 2 tissues of HCC patients using quantitative PCR, Western blot, and immunohistochemistry Chaoshuang Lin1 1,2 assays. The relationship between CCT6A expression and survival was analyzed from the Yuehua Huang GEPIA database and confirmed by immunohistochemistry assays of 133 HCC tissue sec- 1Department of Infectious Diseases, The tions. In addition, the effect of depleting CCT6A on cell proliferation was assessed by CCK- fi Third Af liated Hospital of Sun Yat-sen 8 and colony formation assays. Cell cycle analysis, immunofluorescence assays, GSEA University, Guangzhou 510630, Guangdong, People’s Republic of China; analysis, and cyclin D expression analyzed by Western blot were used to explore the possible 2Guangdong Provincial Key Laboratory of underlying mechanism how dysregulated CCT6A affect the proliferation of HCC. -

Open Full Page
Published OnlineFirst February 10, 2017; DOI: 10.1158/2159-8290.CD-16-1045 RESEARCH ARTICLE Interaction Landscape of Inherited Polymorphisms with Somatic Events in Cancer Hannah Carter 1 , 2 , 3 , 4 , Rachel Marty 5 , Matan Hofree 6 , Andrew M. Gross 5 , James Jensen 5 , Kathleen M. Fisch1,2,3,7 , Xingyu Wu 2 , Christopher DeBoever 5 , Eric L. Van Nostrand 4,8 , Yan Song 4,8 , Emily Wheeler 4,8 , Jason F. Kreisberg 1,3 , Scott M. Lippman 2 , Gene W. Yeo 4,8 , J. Silvio Gutkind 2 , 3 , and Trey Ideker 1 , 2 , 3 , 4 , 5,6 Downloaded from cancerdiscovery.aacrjournals.org on September 27, 2021. © 2017 American Association for Cancer Research. Published OnlineFirst February 10, 2017; DOI: 10.1158/2159-8290.CD-16-1045 ABSTRACT Recent studies have characterized the extensive somatic alterations that arise dur- ing cancer. However, the somatic evolution of a tumor may be signifi cantly affected by inherited polymorphisms carried in the germline. Here, we analyze genomic data for 5,954 tumors to reveal and systematically validate 412 genetic interactions between germline polymorphisms and major somatic events, including tumor formation in specifi c tissues and alteration of specifi c cancer genes. Among germline–somatic interactions, we found germline variants in RBFOX1 that increased incidence of SF3B1 somatic mutation by 8-fold via functional alterations in RNA splicing. Similarly, 19p13.3 variants were associated with a 4-fold increased likelihood of somatic mutations in PTEN. In support of this associ- ation, we found that PTEN knockdown sensitizes the MTOR pathway to high expression of the 19p13.3 gene GNA11 . -

CCT6A Rabbit Pab
Leader in Biomolecular Solutions for Life Science CCT6A Rabbit pAb Catalog No.: A3589 1 Publications Basic Information Background Catalog No. The protein encoded by this gene is a molecular chaperone that is a member of the A3589 chaperonin containing TCP1 complex (CCT), also known as the TCP1 ring complex (TRiC). This complex consists of two identical stacked rings, each containing eight different Observed MW proteins. Unfolded polypeptides enter the central cavity of the complex and are folded 58kDa in an ATP-dependent manner. The complex folds various proteins, including actin and tubulin. Alternate transcriptional splice variants of this gene, encoding different Calculated MW isoforms, have been characterized. In addition, several pseudogenes of this gene have 53kDa/58kDa been located. Category Primary antibody Applications WB,IHC Cross-Reactivity Human, Mouse, Rat Recommended Dilutions Immunogen Information WB 1:500 - 1:2000 Gene ID Swiss Prot 908 P40227 IHC 1:50 - 1:100 Immunogen Recombinant fusion protein containing a sequence corresponding to amino acids 80-250 of human CCT6A (NP_001753.1). Synonyms CCT6A;CCT-zeta;CCT-zeta-1;CCT6;Cctz;HTR3;MoDP-2;TCP-1-zeta;TCP20;TCPZ;TTCP20 Contact Product Information www.abclonal.com Source Isotype Purification Rabbit IgG Affinity purification Storage Store at -20℃. Avoid freeze / thaw cycles. Buffer: PBS with 0.02% sodium azide,50% glycerol,pH7.3. Validation Data Western blot analysis of extracts of various cell lines, using CCT6A antibody (A3589) at 1:1000 dilution. Secondary antibody: HRP Goat Anti-Rabbit IgG (H+L) (AS014) at 1:10000 dilution. Lysates/proteins: 25ug per lane. Blocking buffer: 3% nonfat dry milk in TBST. -

A High-Throughput Approach to Uncover Novel Roles of APOBEC2, a Functional Orphan of the AID/APOBEC Family
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2018 A High-Throughput Approach to Uncover Novel Roles of APOBEC2, a Functional Orphan of the AID/APOBEC Family Linda Molla Follow this and additional works at: https://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons A HIGH-THROUGHPUT APPROACH TO UNCOVER NOVEL ROLES OF APOBEC2, A FUNCTIONAL ORPHAN OF THE AID/APOBEC FAMILY A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy by Linda Molla June 2018 © Copyright by Linda Molla 2018 A HIGH-THROUGHPUT APPROACH TO UNCOVER NOVEL ROLES OF APOBEC2, A FUNCTIONAL ORPHAN OF THE AID/APOBEC FAMILY Linda Molla, Ph.D. The Rockefeller University 2018 APOBEC2 is a member of the AID/APOBEC cytidine deaminase family of proteins. Unlike most of AID/APOBEC, however, APOBEC2’s function remains elusive. Previous research has implicated APOBEC2 in diverse organisms and cellular processes such as muscle biology (in Mus musculus), regeneration (in Danio rerio), and development (in Xenopus laevis). APOBEC2 has also been implicated in cancer. However the enzymatic activity, substrate or physiological target(s) of APOBEC2 are unknown. For this thesis, I have combined Next Generation Sequencing (NGS) techniques with state-of-the-art molecular biology to determine the physiological targets of APOBEC2. Using a cell culture muscle differentiation system, and RNA sequencing (RNA-Seq) by polyA capture, I demonstrated that unlike the AID/APOBEC family member APOBEC1, APOBEC2 is not an RNA editor. Using the same system combined with enhanced Reduced Representation Bisulfite Sequencing (eRRBS) analyses I showed that, unlike the AID/APOBEC family member AID, APOBEC2 does not act as a 5-methyl-C deaminase. -

New Insights on Human Essential Genes Based on Integrated Multi
bioRxiv preprint doi: https://doi.org/10.1101/260224; this version posted February 5, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. New insights on human essential genes based on integrated multi- omics analysis Hebing Chen1,2, Zhuo Zhang1,2, Shuai Jiang 1,2, Ruijiang Li1, Wanying Li1, Hao Li1,* and Xiaochen Bo1,* 1Beijing Institute of Radiation Medicine, Beijing 100850, China. 2 Co-first author *Correspondence: [email protected]; [email protected] Abstract Essential genes are those whose functions govern critical processes that sustain life in the organism. Comprehensive understanding of human essential genes could enable breakthroughs in biology and medicine. Recently, there has been a rapid proliferation of technologies for identifying and investigating the functions of human essential genes. Here, according to gene essentiality, we present a global analysis for comprehensively and systematically elucidating the genetic and regulatory characteristics of human essential genes. We explain why these genes are essential from the genomic, epigenomic, and proteomic perspectives, and we discuss their evolutionary and embryonic developmental properties. Importantly, we find that essential human genes can be used as markers to guide cancer treatment. We have developed an interactive web server, the Human Essential Genes Interactive Analysis Platform (HEGIAP) (http://sysomics.com/HEGIAP/), which integrates abundant analytical tools to give a global, multidimensional interpretation of gene essentiality. bioRxiv preprint doi: https://doi.org/10.1101/260224; this version posted February 5, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

CCT6A Suppresses SMAD2 and Promotes Prometastatic TGF-Β Signaling
The Journal of Clinical Investigation RESEARCH ARTICLE CCT6A suppresses SMAD2 and promotes prometastatic TGF-β signaling Zhe Ying,1,2 Han Tian,1,2 Yun Li,1,2 Rong Lian,1,2 Wei Li,1,2 Shanshan Wu,1,2 Hui-Zhong Zhang,3 Jueheng Wu,1,2 Lei Liu,1,2 Junwei Song,2,4 Hongyu Guan,5 Junchao Cai,1,2 Xun Zhu,1,2 Jun Li,2,4 and Mengfeng Li1,2 1Department of Microbiology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong, China. 2Key Laboratory of Tropical Disease Control, Sun Yat-sen University, Chinese Ministry of Education, Guangzhou, Guangdong, China. 3Department of Cardiothoracic Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China. 4Department of Biochemistry, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong, China. 5Department of Endocrinology and Diabetes Center, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China. Paradoxically, during early tumor development in many cancer types, TGF-β acts as a tumor suppressor, whereas in the advanced stages of these cancers, increased TGF-β expression is linked to high metastasis and poor prognosis. These findings suggest that unidentified mechanisms may function to rewire TGF-β signaling toward its prometastatic role in cancer cells. Our current study using non–small-cell lung carcinoma (NSCLC) cell lines, animal models, and clinical specimens demonstrates that suppression of SMAD2, with SMAD3 function intact, switches TGF-β–induced transcriptional responses to a prometastatic state. Importantly, we identified chaperonin containing TCP1 subunit 6A (CCT6A) as an inhibitor and direct binding protein of SMAD2 and found that CCT6A suppresses SMAD2 function in NSCLC cells and promotes metastasis. -

A Unique Collection of Neurodevelopmental Genes
7586 • The Journal of Neuroscience, August 17, 2005 • 25(33):7586–7600 Development/Plasticity/Repair Telencephalic Embryonic Subtractive Sequences: A Unique Collection of Neurodevelopmental Genes Alessandro Bulfone,2 Pietro Carotenuto,1,3* Andrea Faedo,2* Veruska Aglio,1* Livia Garzia,1,3 Anna Maria Bello,1 Andrea Basile,2 Alessandra Andre`,1 Massimo Cocchia,3 Ombretta Guardiola,1 Andrea Ballabio,3 John L. R. Rubenstein,4 and Massimo Zollo1 1Centro di Ingegneria Genetica e Biotecnologie Avanzate, 80145 Napoli, Italy, 2Stem Cell Research Institute–Hospital San Raffaele, Istituto Scientifico San Raffaele, 20132 Milan, Italy, 3Telethon Institute of Genetics and Medicine, 80131 Naples, Italy, and 4Nina Ireland Laboratory of Developmental Neurobiology, Center for Neurobiology and Psychiatry Genetics, University of California at San Francisco, San Francisco, California 94143-2611 The vertebrate telencephalon is composed of many architectonically and functionally distinct areas and structures, with billions of neurons that are precisely connected. This complexity is fine-tuned during development by numerous genes. To identify genes involved in the regulation of telencephalic development, a specific subset of differentially expressed genes was characterized. Here, we describe a set of cDNAs encoded by genes preferentially expressed during development of the mouse telencephalon that was identified through a functional genomics approach. Of 832 distinct transcripts found, 223 (27%) are known genes. Of the remaining, 228 (27%) correspond to expressed sequence tags of unknown function, 58 (7%) are homologs or orthologs of known genes, and 323 (39%) correspond to novel rare transcripts, including 48 (14%) new putative noncoding RNAs. As an example of this latter group of novel precursor transcripts of micro-RNAs, telencephalic embryonic subtractive sequence (TESS) 24.E3 was functionally characterized, and one of its targets was identified: the zinc finger transcription factor ZFP9. -

CCT6A Suppresses SMAD2 and Promotes Prometastatic TGF-Β Signaling
CCT6A suppresses SMAD2 and promotes prometastatic TGF-β signaling Zhe Ying, … , Jun Li, Mengfeng Li J Clin Invest. 2017;127(5):1725-1740. https://doi.org/10.1172/JCI90439. Research Article Oncology Paradoxically, during early tumor development in many cancer types, TGF-β acts as a tumor suppressor, whereas in the advanced stages of these cancers, increased TGF-β expression is linked to high metastasis and poor prognosis. These findings suggest that unidentified mechanisms may function to rewire TGF-β signaling toward its prometastatic role in cancer cells. Our current study using non–small-cell lung carcinoma (NSCLC) cell lines, animal models, and clinical specimens demonstrates that suppression of SMAD2, with SMAD3 function intact, switches TGF-β–induced transcriptional responses to a prometastatic state. Importantly, we identified chaperonin containing TCP1 subunit 6A (CCT6A) as an inhibitor and direct binding protein of SMAD2 and found that CCT6A suppresses SMAD2 function in NSCLC cells and promotes metastasis. Furthermore, selective inhibition of SMAD3 or CCT6A efficiently suppresses TGF-β–mediated metastasis. Our findings provide a mechanism that directs TGF-β signaling toward its prometastatic arm and may contribute to the development of therapeutic strategies targeting TGF-β for NSCLC. Find the latest version: https://jci.me/90439/pdf The Journal of Clinical Investigation RESEARCH ARTICLE CCT6A suppresses SMAD2 and promotes prometastatic TGF-β signaling Zhe Ying,1,2 Han Tian,1,2 Yun Li,1,2 Rong Lian,1,2 Wei Li,1,2 Shanshan Wu,1,2 Hui-Zhong Zhang,3 Jueheng Wu,1,2 Lei Liu,1,2 Junwei Song,2,4 Hongyu Guan,5 Junchao Cai,1,2 Xun Zhu,1,2 Jun Li,2,4 and Mengfeng Li1,2 1Department of Microbiology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong, China. -

Heat Shock Proteins Create a Signature to Predict the Clinical Outcome In
www.nature.com/scientificreports OPEN Heat shock proteins create a signature to predict the clinical outcome in breast cancer Received: 16 July 2018 Marta Klimczak1,2, Przemyslaw Biecek3,4, Alicja Zylicz 1 & Maciej Zylicz1 Accepted: 27 April 2019 Utilizing The Cancer Genome Atlas (TCGA) and KM plotter databases we identifed six heat shock Published: xx xx xxxx proteins associated with survival of breast cancer patients. The survival curves of samples with high and low expression of heat shock genes were compared by log-rank test (Mantel-Haenszel). Interestingly, patients overexpressing two identifed HSPs – HSPA2 and DNAJC20 exhibited longer survival, whereas overexpression of other four HSPs – HSP90AA1, CCT1, CCT2, CCT6A resulted in unfavorable prognosis for breast cancer patients. We explored correlations between expression level of HSPs and clinicopathological features including tumor grade, tumor size, number of lymph nodes involved and hormone receptor status. Additionally, we identifed a novel signature with the potential to serve as a prognostic model for breast cancer. Using univariate Cox regression analysis followed by multivariate Cox regression analysis, we built a risk score formula comprising prognostic HSPs (HSPA2, DNAJC20, HSP90AA1, CCT1, CCT2) and tumor stage to identify high-risk and low-risk cases. Finally, we analyzed the association of six prognostic HSP expression with survival of patients sufering from other types of cancer than breast cancer. We revealed that depending on cancer type, each of the six analyzed HSPs can act both as a positive, as well as a negative regulator of cancer development. Our study demonstrates a novel HSP signature for the outcome prediction of breast cancer patients and provides a new insight into ambiguous role of these proteins in cancer development. -

Gene Amplifications at Chromosome 7 of the Human Gastric Cancer Genome
225-231 4/7/07 20:50 Page 225 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 20: 225-231, 2007 225 Gene amplifications at chromosome 7 of the human gastric cancer genome SANGHWA YANG Cancer Metastasis Research Center, Yonsei University College of Medicine, 134 Shinchon-Dong, Seoul 120-752, Korea Received April 20, 2007; Accepted May 7, 2007 Abstract. Genetic aberrations at chromosome 7 are known to Introduction be related with diverse human diseases, including cancer and autism. In a number of cancer research areas involving gastric The completion of human genome sequencing and the cancer, several comparative genomic hybridization studies subsequent gene annotations, together with a rapid develop- employing metaphase chromosome or BAC clone micro- ment of high throughput screening technologies, such as DNA arrays have repeatedly identified human chromosome 7 microarrays, have made it possible to perform genome-scale as containing ‘regions of changes’ related with cancer expression profiling and comparative genomic hybridizations progression. cDNA microarray-based comparative genomic (CGHs) in various cancer models. The elucidation of gene hybridization can be used to directly identify individual target copy number variations in several cancer genomes is generating genes undergoing copy number variations. Copy number very informative results. Metaphase chromosome CGH and change analysis for 17,000 genes on a microarray format was the recent introduction of BAC and especially cDNA micro- performed with tumor and normal gastric tissues from 30 array-based CGHs (aCGH) (1) have greatly contributed to patients. A group of 90 genes undergoing copy number the identification of chromosome aberrations and of amplified increases (gene amplification) at the p11~p22 or q21~q36 and deleted genes in gastric cancer tissues and cell lines (2-9).