Table S1. ALS Proteomic Studies Used in the Comparison. Ref ALS Model/Sample Type Differentially Expressed Proteins Proteins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Screening and Identification of Key Biomarkers in Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Screening and identification of key biomarkers in clear cell renal cell carcinoma based on bioinformatics analysis Basavaraj Vastrad1, Chanabasayya Vastrad*2 , Iranna Kotturshetti 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. 3. Department of Ayurveda, Rajiv Gandhi Education Society`s Ayurvedic Medical College, Ron, Karnataka 562209, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Clear cell renal cell carcinoma (ccRCC) is one of the most common types of malignancy of the urinary system. The pathogenesis and effective diagnosis of ccRCC have become popular topics for research in the previous decade. In the current study, an integrated bioinformatics analysis was performed to identify core genes associated in ccRCC. An expression dataset (GSE105261) was downloaded from the Gene Expression Omnibus database, and included 26 ccRCC and 9 normal kideny samples. Assessment of the microarray dataset led to the recognition of differentially expressed genes (DEGs), which was subsequently used for pathway and gene ontology (GO) enrichment analysis. -

Cyclin D1/Cyclin-Dependent Kinase 4 Interacts with Filamin a and Affects the Migration and Invasion Potential of Breast Cancer Cells
Published OnlineFirst February 28, 2010; DOI: 10.1158/0008-5472.CAN-08-1108 Tumor and Stem Cell Biology Cancer Research Cyclin D1/Cyclin-Dependent Kinase 4 Interacts with Filamin A and Affects the Migration and Invasion Potential of Breast Cancer Cells Zhijiu Zhong, Wen-Shuz Yeow, Chunhua Zou, Richard Wassell, Chenguang Wang, Richard G. Pestell, Judy N. Quong, and Andrew A. Quong Abstract Cyclin D1 belongs to a family of proteins that regulate progression through the G1-S phase of the cell cycle by binding to cyclin-dependent kinase (cdk)-4 to phosphorylate the retinoblastoma protein and release E2F transcription factors for progression through cell cycle. Several cancers, including breast, colon, and prostate, overexpress the cyclin D1 gene. However, the correlation of cyclin D1 overexpression with E2F target gene regulation or of cdk-dependent cyclin D1 activity with tumor development has not been identified. This suggests that the role of cyclin D1 in oncogenesis may be independent of its function as a cell cycle regulator. One such function is the role of cyclin D1 in cell adhesion and motility. Filamin A (FLNa), a member of the actin-binding filamin protein family, regulates signaling events involved in cell motility and invasion. FLNa has also been associated with a variety of cancers including lung cancer, prostate cancer, melanoma, human bladder cancer, and neuroblastoma. We hypothesized that elevated cyclin D1 facilitates motility in the invasive MDA-MB-231 breast cancer cell line. We show that MDA-MB-231 motility is affected by disturbing cyclin D1 levels or cyclin D1-cdk4/6 kinase activity. -

Supp Material.Pdf
Supplemental Materials Genome-wide Mapping of SMAD Target genes Reveals the Role of BMP Signaling in Embryonic Stem Cell Fate Determination Teng Fei1#, Kai Xia2#, Zhongwei Li1^, Bing Zhou2^, Shanshan Zhu2^, Hua Chen1, Jianping Zhang1, Zhang Chen2, Huasheng Xiao3, Jing-Dong J. Han2*, Ye-Guang Chen1* Supplemental Methods Supplemental References Supplemental Figures S1–S17 Supplemental Tables S1-S9 1 Supplemental Methods Cell culture and antibodies Murine R1 ES cells were first plated on mitomycin-treated murine embryonic fibroblasts (MEFs) and grown under typical ES cell conditions (DMEM supplemented with 15% fetal calf serum, leukemia inhibitory factor (LIF), non-essential amino acids, GlutaMax-I (Invitrogen Gibco), beta-mercaptoethanol and penicillin/streptomycin. For ChIP assay, cells were cultured on gelatinized tissue culture plates for two passages to eliminate MEFs. KO-DMEM and Knockout serum replacement (Invitrogen Gibco) were used to substitute DMEM and fetal calf serum for feeder free culture. For ChIP experiments and immunoblotting, we used affinity-purified SMAD1/5 and SMAD4 antibodies that can recognize a specific band corresponding to endogenous SMAD proteins in R1 ES cells (Fig. S1A). Rabbit anti-SMAD1/5 antibody was generated with recombinant human SMAD1-linker (aa145-267)-His, and rabbit anti-SMAD4 antibody with recombinant human SMAD4-linker (aa144-316)-His. Anti-SMAD1/5 antibody can recognize both overexpressed SMAD1 and SMAD5 proteins but minimally SMAD8 (Fig. S1B). Phospho-SMAD1/5 antibody was purchased from Chemicon International (AB3848) and anti-nestin antibody from Chemicon International (MAB353). H3K27me3 antibody (ab6002) and histone H3 antibody (ab1791) were from Abcam. GFP (SC-8334), GST (SC-138), His (SC-803) antibody were from Santa Cruz Biotech. -

Supplementary Figures
Mena regulates the LINC complex to control actin–nuclear lamina associations, trans-nuclear membrane signalling and cancer gene expression Frederic Li Mow Chee!, Bruno Beernaert!, Alexander Loftus!, Yatendra Kumar", Billie G. C. Griffith!, Jimi C. Wills!, Ann P. Wheeler#, J. Douglas Armstrong$, Maddy Parsons%, Irene M. Leigh,(, Charlotte M. Proby&, Alex von Kriegsheim!, Wendy A. Bickmore", Margaret C. Frame,* & Adam Byron,* Supplementary Information Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3 Supplementary Table 1 Supplementary Table 2 Supplementary Table 3 Supplementary Table 4 !Cancer Research UK Edinburgh Centre, Institute of Genetics and Cancer, University of Edinburgh, Edinburgh EH< =XR, UK. "MRC Human Genetics Unit, Institute of Genetics and Cancer, University of Edinburgh, Edinburgh EH< =XU, UK. #Advanced Imaging Resource, Institute of Genetics and Cancer, University of Edinburgh, Edinburgh EH< =XU, UK. $Simons Initiative for the Developing Brain, School of Informatics, University of Edinburgh, Edinburgh EHH IYL, UK. %Randall Centre for Cell and Molecular Biophysics, King’s College London, London SEM MUL, UK. &Division of Molecular and Clinical Medicine, School of Medicine, University of Dundee, Dundee DD <HN, UK. 'Institute of Dentistry, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London EM =AT, UK. *email: [email protected] or [email protected] 1 a cSCC IAC correlation b cSCC IAC pathways c Core adhesome network ENAH −log10(q) MACF1 CSRP1 Met1 Met4 0 5 10 + + CORO2A Integrin signalling + CFL1 pathway PRNP ILK + HSPB1 PALLD PPFIA1 TES RDX Cytoskeletal regulation + VASP + + ARPC2 by Rho GTPase PPP2CA + Met1 + LASP1 MYH9 + VIM TUBA4A Huntington ITGA3 + disease ITGB4 VCL CAV1 ACTB ROCK1 KTN1 FLNA+ CALR DNA FBLIM1 CORO1B RAC1 + replication +ACTN1 ITGA6 + Met4 ITGAV Parkinson ITGB1 disease Actin cytoskel. -

Protein Interaction Network of Alternatively Spliced Isoforms from Brain Links Genetic Risk Factors for Autism
ARTICLE Received 24 Aug 2013 | Accepted 14 Mar 2014 | Published 11 Apr 2014 DOI: 10.1038/ncomms4650 OPEN Protein interaction network of alternatively spliced isoforms from brain links genetic risk factors for autism Roser Corominas1,*, Xinping Yang2,3,*, Guan Ning Lin1,*, Shuli Kang1,*, Yun Shen2,3, Lila Ghamsari2,3,w, Martin Broly2,3, Maria Rodriguez2,3, Stanley Tam2,3, Shelly A. Trigg2,3,w, Changyu Fan2,3, Song Yi2,3, Murat Tasan4, Irma Lemmens5, Xingyan Kuang6, Nan Zhao6, Dheeraj Malhotra7, Jacob J. Michaelson7,w, Vladimir Vacic8, Michael A. Calderwood2,3, Frederick P. Roth2,3,4, Jan Tavernier5, Steve Horvath9, Kourosh Salehi-Ashtiani2,3,w, Dmitry Korkin6, Jonathan Sebat7, David E. Hill2,3, Tong Hao2,3, Marc Vidal2,3 & Lilia M. Iakoucheva1 Increased risk for autism spectrum disorders (ASD) is attributed to hundreds of genetic loci. The convergence of ASD variants have been investigated using various approaches, including protein interactions extracted from the published literature. However, these datasets are frequently incomplete, carry biases and are limited to interactions of a single splicing isoform, which may not be expressed in the disease-relevant tissue. Here we introduce a new interactome mapping approach by experimentally identifying interactions between brain-expressed alternatively spliced variants of ASD risk factors. The Autism Spliceform Interaction Network reveals that almost half of the detected interactions and about 30% of the newly identified interacting partners represent contribution from splicing variants, emphasizing the importance of isoform networks. Isoform interactions greatly contribute to establishing direct physical connections between proteins from the de novo autism CNVs. Our findings demonstrate the critical role of spliceform networks for translating genetic knowledge into a better understanding of human diseases. -

The Myotonic Dystrophy Locus
J Med Genet: first published as 10.1136/jmg.28.2.84 on 1 February 1991. Downloaded from 848 Med Genet 1991; 28: 84-88 Identification of new DNA markers close to the myotonic dystrophy locus J D Brook, H G Harley, K V Walsh, S A Rundle, M J Siciliano, P S Harper, D J Shaw Abstract dominant condition with a prevalence of 1 in 8000. The most useful markers for the prenatal diagnosis The DM gene is located on the long arm of of myotonic dystrophy (DM) are APOC2 and chromosome 19 within the interval 19q13.2-19q13.3. CKM, both of which map proximal to DM. In order Recently, a consensus order for markers in this region to produce other markers useful for DM, we have has emerged, which reads from centromere to telo- screened genomic DNA libraries constructed from mere: BCL3-APOC2-CKM-ERCCI-DM-D19SSO- cell line 20XP3542-1-4, which contains 20 to 30 Mb D19S22.25 On the proximal side of DM, CKM and of human material including APOC2 and CKM. ERCCI map to the same 300 kb NotI fragment.6 For Of 51 human clones identified, seven map to ERCCI few linkage data are available, as no con- chromosome 17, four to chromosome 8, and nine to ventional RFLPs have been documented. CKM chromosome 19, and the remaining 31 were excluded is closely linked to DM with a Omax of 0-02 (Zmax= from chromosome 19 but not localised further. 22 20) and a -1 lod support interval of 0 to 0 05. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Proteomics Provides Insights Into the Inhibition of Chinese Hamster V79
www.nature.com/scientificreports OPEN Proteomics provides insights into the inhibition of Chinese hamster V79 cell proliferation in the deep underground environment Jifeng Liu1,2, Tengfei Ma1,2, Mingzhong Gao3, Yilin Liu4, Jun Liu1, Shichao Wang2, Yike Xie2, Ling Wang2, Juan Cheng2, Shixi Liu1*, Jian Zou1,2*, Jiang Wu2, Weimin Li2 & Heping Xie2,3,5 As resources in the shallow depths of the earth exhausted, people will spend extended periods of time in the deep underground space. However, little is known about the deep underground environment afecting the health of organisms. Hence, we established both deep underground laboratory (DUGL) and above ground laboratory (AGL) to investigate the efect of environmental factors on organisms. Six environmental parameters were monitored in the DUGL and AGL. Growth curves were recorded and tandem mass tag (TMT) proteomics analysis were performed to explore the proliferative ability and diferentially abundant proteins (DAPs) in V79 cells (a cell line widely used in biological study in DUGLs) cultured in the DUGL and AGL. Parallel Reaction Monitoring was conducted to verify the TMT results. γ ray dose rate showed the most detectable diference between the two laboratories, whereby γ ray dose rate was signifcantly lower in the DUGL compared to the AGL. V79 cell proliferation was slower in the DUGL. Quantitative proteomics detected 980 DAPs (absolute fold change ≥ 1.2, p < 0.05) between V79 cells cultured in the DUGL and AGL. Of these, 576 proteins were up-regulated and 404 proteins were down-regulated in V79 cells cultured in the DUGL. KEGG pathway analysis revealed that seven pathways (e.g. -
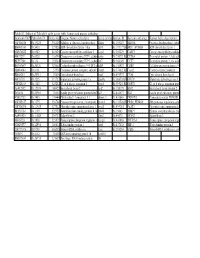
Table S3: Subset of Zebrafish Early Genes with Human And
Table S3: Subset of Zebrafish early genes with human and mouse orthologs Genbank ID(ZFZebrafish ID Entrez GenUnigene Name (zebrafish) Gene symbo Human ID Humann ortholog Human Gene description AW116838 Dr.19225 336425 Aldolase a, fructose-bisphosphate aldoa Hs.155247 ALDOA Fructose-bisphosphate aldola BM005100 Dr.5438 327026 ADP-ribosylation factor 1 like arf1l Hs.119177||HsARF1_HUMAN ADP-ribosylation factor 1 AW076882 Dr.6582 403025 Cancer susceptibility candidate 3 casc3 Hs.350229 CASC3 Cancer susceptibility candidat AI437239 Dr.6928 116994 Chaperonin containing TCP1, subun cct6a Hs.73072||Hs.CCT6A T-complex protein 1, zeta sub BE557308 Dr.134 192324 Chaperonin containing TCP1, subun cct7 Hs.368149 CCT7 T-complex protein 1, eta subu BG303647 Dr.26326 321602 Cyclin-dependent kinase 9 (CDC2-recdk9 Hs.150423 CDK9 Cell division protein kinase 9 AB040044 Dr.8161 57970 Coatomer protein complex, subunit zcopz1 Hs.37482||Hs.Copz2 Coatomer zeta-2 subunit BI888253 Dr.20911 30436 Eyes absent homolog 1 eya1 Hs.491997 EYA4 Eyes absent homolog 4 AI878758 Dr.3225 317737 Glutamate dehydrogenase 1a glud1a Hs.368538||HsGLUD1 Glutamate dehydrogenase 1, AW128619 Dr.1388 325284 G1 to S phase transition 1 gspt1 Hs.59523||Hs.GSPT1 G1 to S phase transition prote AF412832 Dr.12595 140427 Heat shock factor 2 hsf2 Hs.158195 HSF2 Heat shock factor protein 2 D38454 Dr.20916 30151 Insulin gene enhancer protein Islet3 isl3 Hs.444677 ISL2 Insulin gene enhancer protein AY052752 Dr.7485 170444 Pbx/knotted 1 homeobox 1.1 pknox1.1 Hs.431043 PKNOX1 Homeobox protein PKNOX1 -

Genetic and Genomic Analysis of Hyperlipidemia, Obesity and Diabetes Using (C57BL/6J × TALLYHO/Jngj) F2 Mice
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Nutrition Publications and Other Works Nutrition 12-19-2010 Genetic and genomic analysis of hyperlipidemia, obesity and diabetes using (C57BL/6J × TALLYHO/JngJ) F2 mice Taryn P. Stewart Marshall University Hyoung Y. Kim University of Tennessee - Knoxville, [email protected] Arnold M. Saxton University of Tennessee - Knoxville, [email protected] Jung H. Kim Marshall University Follow this and additional works at: https://trace.tennessee.edu/utk_nutrpubs Part of the Animal Sciences Commons, and the Nutrition Commons Recommended Citation BMC Genomics 2010, 11:713 doi:10.1186/1471-2164-11-713 This Article is brought to you for free and open access by the Nutrition at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Nutrition Publications and Other Works by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. Stewart et al. BMC Genomics 2010, 11:713 http://www.biomedcentral.com/1471-2164/11/713 RESEARCH ARTICLE Open Access Genetic and genomic analysis of hyperlipidemia, obesity and diabetes using (C57BL/6J × TALLYHO/JngJ) F2 mice Taryn P Stewart1, Hyoung Yon Kim2, Arnold M Saxton3, Jung Han Kim1* Abstract Background: Type 2 diabetes (T2D) is the most common form of diabetes in humans and is closely associated with dyslipidemia and obesity that magnifies the mortality and morbidity related to T2D. The genetic contribution to human T2D and related metabolic disorders is evident, and mostly follows polygenic inheritance. The TALLYHO/ JngJ (TH) mice are a polygenic model for T2D characterized by obesity, hyperinsulinemia, impaired glucose uptake and tolerance, hyperlipidemia, and hyperglycemia. -

Evidence for a Tumor Suppressor Gene on Chromosome 19Q Associated with Human Astrocytomas, Oligodendrogliomas, and Mixed Gliomas1
(CANCER RESEARCH 52. 4277-4279, August 1. 1992| Advances in Brief Evidence for a Tumor Suppressor Gene on Chromosome 19q Associated with Human Astrocytomas, Oligodendrogliomas, and Mixed Gliomas1 Andreas von Deimling,2 David N. Louis,3 Klaus von Ammon, Iver Petersen, Otmar D. Wiestier, and Bernd R. Seizinger Molecular Neuro-Oncology Laboratory and Neurosurgical Service, Massachusetts General Hospital and Harvard Medical School, Charlestown, Massachusetts 02129 [A. v. D., D. N. L,, B. R. S.J; Department of Pathology (Neuropathology), Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114 ¡D.N. L.J; Department of Neurosurgery, University of Zürich,Raemistrasse 100, CH-8091 Zürich,Switzerland ¡K.v. A.]; and Laboratory of Neuropathology, Department of Pathology, University of Zurich, Schmelzbergstrasse 12, CH-8091 Zürich,Switzerland [L P., O. D. W.¡ Abstract is unique to fibrillary astrocytomas and Oligodendrogliomas, and whether a putative tumor suppressor gene locus exists on Previous studies have shown frequent allelic losses of chromosomes the long arm of chromosome 19, we evaluated 122 glial tumors 9p, 10, 17p, and 22q in glial tumors. Other researchers have briefly for LOH events on chromosome 19. reported that glial tumors may also show allelic losses of chromosome 19, suggesting a putative tumor suppressor gene locus on this chromo Materials and Methods some (D. T. Ransom et al., Proc. Am. Assoc. Cancer Res., 32: 302, 1991). To evaluate whether loss of chromosome 19 alÃelesis common in Tissue Specimens and Histopathology. Tumor and blood samples glial tumors of different types and grades, we performed Southern blot were obtained from 116 patients treated at the Massachusetts General restriction fragment length polymorphism analysis for multiple chro Hospital (Boston, MA) and at the University Hospital (Zurich, Swit mosome 19 loci in 122 gliomas from 116 patients. -
The Endogenous Transposable Element Tgm9 Is Suitable for Generating Knockout Mutants for Functional Analyses of Soybean Genes and Genetic Improvement in Soybean
RESEARCH ARTICLE The endogenous transposable element Tgm9 is suitable for generating knockout mutants for functional analyses of soybean genes and genetic improvement in soybean Devinder Sandhu1*, Jayadri Ghosh2, Callie Johnson3, Jordan Baumbach2, Eric Baumert3, Tyler Cina3, David Grant2,4, Reid G. Palmer2,4², Madan K. Bhattacharyya2* a1111111111 1 USDA-ARS, US Salinity Laboratory, Riverside, CA, United States of America, 2 Department of Agronomy, a1111111111 Iowa State University, Ames, IA, United States of America, 3 Department of Biology, University of Wisconsin- a1111111111 Stevens Point, Stevens Point, WI, United States of America, 4 USDA-ARS Corn Insects and Crop Genomics a1111111111 Research Unit, Ames, IA, United States of America a1111111111 ² Deceased. * [email protected] (DS); [email protected] (MKB) OPEN ACCESS Abstract Citation: Sandhu D, Ghosh J, Johnson C, In soybean, variegated flowers can be caused by somatic excision of the CACTA-type trans- Baumbach J, Baumert E, Cina T, et al. (2017) The posable element Tgm9 from Intron 2 of the DFR2 gene encoding dihydroflavonol-4-reduc- endogenous transposable element Tgm9 is suitable for generating knockout mutants for tase of the anthocyanin pigment biosynthetic pathway. DFR2 was mapped to the W4 locus, functional analyses of soybean genes and genetic where the allele containing Tgm9 was termed w4-m. In this study we have demonstrated improvement in soybean. PLoS ONE 12(8): that previously identified morphological mutants (three chlorophyll deficient mutants, one e0180732. https://doi.org/10.1371/journal. male sterile-female fertile mutant, and three partial female sterile mutants) were caused by pone.0180732 insertion of Tgm9 following its excision from DFR2.