Catalytic Reforming
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(HDS) Unit for Petroleum Naphtha at 3500 Barrels Per Day
Available online at www.worldscientificnews.com WSN 9 (2015) 88-100 EISSN 2392-2192 Design Parameters for a Hydro desulfurization (HDS) Unit for Petroleum Naphtha at 3500 Barrels per Day Debajyoti Bose University of Petroleum & Energy Studies, College of Engineering Studies, P.O. Bidholi via- Prem Nagar, Dehradun 248007, India E-mail address: [email protected] ABSTRACT The present work reviews the setting up of a hydrodesulphurization unit for petroleum naphtha. Estimating all the properties of the given petroleum fraction including its density, viscosity and other parameters. The process flow sheet which gives the idea of necessary equipment to be installed, then performing all material and energy balance calculations along with chemical and mechanical design for the entire setup taking into account every instrument considered. The purpose of this review paper takes involves an industrial process, a catalytic chemical process widely used to remove sulfur (S) from naphtha. Keywords: hydro desulfurization, naphtha, petroleum, sulfur Relevance to Design Practice - The purpose of removing the sulfur is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion. World Scientific News 9 (2015) 88-100 1. INTRODUCTION Hydrodesulphurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur is to reduce the sulfur dioxide (SO2) emissions that result from various combustion practices. -

BENZENE Disclaimer
United States Office of Air Quality EPA-454/R-98-011 Environmental Protection Planning And Standards June 1998 Agency Research Triangle Park, NC 27711 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF BENZENE Disclaimer This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Mention of trade names and commercial products does not constitute endorsement or recommendation of use. EPA-454/R-98-011 ii TABLE OF CONTENTS Section Page LIST OF TABLES.....................................................x LIST OF FIGURES.................................................. xvi EXECUTIVE SUMMARY.............................................xx 1.0 PURPOSE OF DOCUMENT .......................................... 1-1 2.0 OVERVIEW OF DOCUMENT CONTENTS.............................. 2-1 3.0 BACKGROUND INFORMATION ...................................... 3-1 3.1 NATURE OF POLLUTANT..................................... 3-1 3.2 OVERVIEW OF PRODUCTION AND USE ......................... 3-4 3.3 OVERVIEW OF EMISSIONS.................................... 3-8 4.0 EMISSIONS FROM BENZENE PRODUCTION ........................... 4-1 4.1 CATALYTIC REFORMING/SEPARATION PROCESS................ 4-7 4.1.1 Process Description for Catalytic Reforming/Separation........... 4-7 4.1.2 Benzene Emissions from Catalytic Reforming/Separation .......... 4-9 4.2 TOLUENE DEALKYLATION AND TOLUENE DISPROPORTIONATION PROCESS ............................ 4-11 4.2.1 Toluene Dealkylation -

Optimize Refinery Hydrotreating & Catalytic Reforming Using Fast On
— ABB MEASUREMENT & ANALYTICS | APPlicatiON NOte Optimize refinery hydrotreating & catalytic reforming Using fast on-line simulated distillation GCs PGC5009 fast gas chromatograph The advantage of on-line fast simulated distillation. Measurement made easy — The challenge Catalytic reforming is a major conversion process in Industry | Refining Improve your control Improving profitability requires refiners to reduce petroleum refinery and petrochemical industries. strategies. the impact of market price volatility and product The process converts low octane naphthas into environmental compliance requirements by higher octane reformate products for gasoline adopting better control strategies. Better control blending and aromatic rich reformate for aromatic strategies enable refiners to handle a wide range of production. Hydrotreating is an important step to feedstocks while maintaining smooth operations remove unwanted sulfur from streams that are used and intermediate product qualities, both key to as feeds to some refinery units. Catalytic reforming sustainable margins and reliable operation. Most and isomerization are examples of refining traditional process and lab gas chromatographs as processes that require low sulfur feeds. The catalyst well as D86 distillation devices do not provide the in these processes are platinum based and sulfur required fast and reliable measurement feedback compounds are catalyst poisons. Controlling the due to long cycle times and poor repeatability. feed distillation properties enable control on not only the product properties but also the monitoring The ABB PGC5009 fast on-line gas chromatograph of the sulfur compounds. Intermediate streams used answers the challenge by providing superior process to feed hydrotreater do not have a uniform amount chromatography for simulate distillation in less than of sulfur compounds across their boiling point range 5 minutes. -

Simulation and Modeling of Catalytic Reforming Process
Petroleum & Coal ISSN 1337-7027 Available online at www.vurup.sk/petroleum-coal Petroleum & Coal 54 (1) 76-84, 2012 SIMULATION AND MODELING OF CATALYTIC REFORMING PROCESS Aboalfazl Askari*, Hajir Karimi, M.Reza Rahimi, Mehdi Ghanbari Chemical engineering department, School of engineering,Yasouj University,Yasouj 75918-74831, Iran; [email protected] Received July 26, 2011, Accepted January 5, 2012 Abstract One of the most important processes in oil refineries is catalytic reforming unit in which high octane gasoline is produced. The catalytic reforming unit by using Hysys-refinery software was simulated. The results are validated by operating data, which is taken from the Esfahan oil refinery catalytic reforming unit. Usually, in oil refineries, flow instability in composition of feedstock can affect the product quality. The attention of this paper was focused on changes of the final product flow rate and product’s octane number with respect to the changes in the feedstock composition. Also, the effects of temperature and pressure on the mentioned parameters was evaluated. Furthermore, in this study, Smith kinetic model was evaluated. The accuracy of this model was compared with the actual data and Hysys-refinery’s results. The results showed that if the feed stream of catalytic reforming unit supplied with the Heavy Isomax Naphtha can be increased, more than 20% of the current value, the flow rate and octane number of the final product will be increased. Also, we found that the variations of temperature and pressure, under operating condition of the reactors of this unit, has no effect on octane number and final product flow rate. -

Reforming for Btx
PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Menlo Park, California Abstract Process Economics Program Report No. 129 REFORMING FOR BTX (June 1980) This report reviews the reforming of naphtha to produce benzene, toluene, and xylenes. The fundamentals of the reforming operations are discussed in detail. The economics were developed for reforming of two naphthas; a paraffinic and a naphthenic. Also, the effect of reforming severity on the economics is studied. LAC SF PEP'77 WS Fw Report No. 129 REFORMING FOR BTX by FRANK B. WEST Contributions by LESLIE A. CARMICHAEL STANFORD FIELD KOON LING RING WALTER SEDRIKS May 1980 A private report by the PROCESS ECONOMICS PROGRAM Menlo Park, California 94025 For detailed marketing data and information, the reader is referred to one of the SRI programs specializing-in marketing research. The CHEMICAL ECONOMICS UANDROOK Program covers most major chemicals and chemical products produced in the United States and the WORLD PETROCHEMICALS Program covers major hydrocarbons and their derivatives on a worldwide basis. In addition, the SRI DIRECTCRY OF CHEMICAL PRODUCERS services provide detailed lists of chemical producers by company, prod- uct, and plant for the United States and Western Europe. ii CONTENTS 1 INTRODUCTION . 1 2 SUMMARY . 3 3 INDUSTRY STATUS . : .................... 9 Production Capacity .................... 9 4 GENERAL PROCESS CONSIDERATIONS ............... 17 Introduction. ....................... 17 Chemistry ......................... 18 General ......................... 18 Feed Pretreating Reactions ................ 20 Reforming Reactions ................... 21 Isomerization and Dehydrogenation of Naphthenes ..... 22 Isomerization and Dehydrocyclization of Paraffins .... 23 Isomerization, Dealkylation, and Disproportionation of Aromatics ...................... 27 Isomerization ...................... 27 0 Dealkylation ....................... 27 Disproportionation and Transalkylation .......... 28 Hydrocracking of Paraffins and Naphthenes ........ 29 Coke Formation on the Catalyst ............. -

5.1 Petroleum Refining1
5.1 Petroleum Refining1 5.1.1 General Description The petroleum refining industry converts crude oil into more than 2500 refined products, including liquefied petroleum gas, gasoline, kerosene, aviation fuel, diesel fuel, fuel oils, lubricating oils, and feedstocks for the petrochemical industry. Petroleum refinery activities start with receipt of crude for storage at the refinery, include all petroleum handling and refining operations and terminate with storage preparatory to shipping the refined products from the refinery. The petroleum refining industry employs a wide variety of processes. A refinery's processing flow scheme is largely determined by the composition of the crude oil feedstock and the chosen slate of petroleum products. The example refinery flow scheme presented in Figure 5.1-1 shows the general processing arrangement used by refineries in the United States for major refinery processes. The arrangement of these processes will vary among refineries, and few, if any, employ all of these processes. Petroleum refining processes having direct emission sources are presented on the figure in bold-line boxes. Listed below are 5 categories of general refinery processes and associated operations: 1. Separation processes a. Atmospheric distillation b. Vacuum distillation c. Light ends recovery (gas processing) 2. Petroleum conversion processes a. Cracking (thermal and catalytic) b. Reforming c. Alkylation d. Polymerization e. Isomerization f. Coking g. Visbreaking 3.Petroleum treating processes a. Hydrodesulfurization b. Hydrotreating c. Chemical sweetening d. Acid gas removal e. Deasphalting 4.Feedstock and product handling a. Storage b. Blending c. Loading d. Unloading 5.Auxiliary facilities a. Boilers b. Waste water treatment c. Hydrogen production d. -
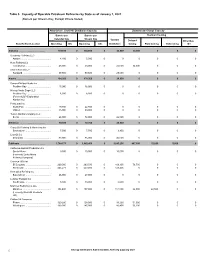
Table 3. Capacity of Operable Petroleum Refineries by State As of January 1, 2021 (Barrels Per Stream Day, Except Where Noted)
Table 3. Capacity of Operable Petroleum Refineries by State as of January 1, 2021 (Barrels per Stream Day, Except Where Noted) Atmospheric Crude Oil Distillation Capacity Downstream Charge Capacity Barrels per Barrels per Thermal Cracking Calendar Day Stream Day Vacuum Delayed Other/Gas State/Refiner/Location Operating Idle Operating Idle Distillation Coking Fluid Coking Visbreaking Oil Alabama......................................................... 139,600 0 145,600 0 54,000 34,000 0 0 0 Goodway Refining LLC ....................................................................................................................................................................................................Atmore 4,100 0 5,000 0 0 0 0 0 0 Hunt Refining Co ....................................................................................................................................................................................................Tuscaloosa 48,000 0 50,000 0 25,000 34,000 0 0 0 Shell Chemical LP ....................................................................................................................................................................................................Saraland 87,500 0 90,600 0 29,000 0 0 0 0 Alaska......................................................... 164,200 0 178,500 0 26,000 0 0 0 0 ConocoPhillips Alaska Inc ....................................................................................................................................................................................................Prudhoe -

Preliminary Data Summary for the Petroleum Refining Category
Preliminary Data Summary for the Petroleum Refining Category United States Environmental Protection Agency Office of Water Engineering and Analysis Division 401 M Street, S.W. Washington, D.C. 20460 EPA 821-R-96-015 Apr il 1996 Table of Contents List of Figures iv List of Tables v Acknowledgments vii 1. Introduction 1 1.1 Background 1 1.2 Status of Categorical Regulations 1 1.3 Software Disk Available 4 2. Description of the Industry 5 2.1 Production Operations 5 2.1.1 Crude Oil and Product Storage 5 2.1.2 Crude Distillation 5 2.1.3 Cracking 6 2.1.4 Hydrocarbon Rebuilding 6 2.1.5 Hydrocarbon Rearrangements 6 2.1.6 Hydrotreating 6 2.1.7 Solvent Refining 7 2.1.8 Asphalt Production 7 2.1.9 Lubricating Oil Manufacture 7 2.1.10 Production of Petrochemicals 8 2.2 Industry Trends 8 3. Summary of Information Sources Used in This Study 13 3.1 Oil And Gas Journal Survey 13 3.2 EPA Office of Air and Radiation Questionnaire 13 3.3 Plant Visits 13 3.4 Permit Compliance System Data 13 3.5 Los Angeles County Sanitation Districts 14 3.6 Other Sewerage Authorities 14 3.7 Province of Ontario, Canada Petroleum Study 14 3.8 Other Data Sources 14 4. Treatment Technologies Used in The Industry 15 4.1 In-Plant Controls 16 4.1.1 Steam Stripping 16 4.1.2 Neutralization of Spent Acids and Caustics 18 4.1.3 Source Control 18 4.1.4 Wastewater Segregation 18 4.1.5 Boiler Condensate Recovery 19 4.1.6 Treated Effluent Reuse 19 i 4.1.7 Other General Measures 19 4.1.8 Cooling Water Systems 19 4.1.9 Once-Through Cooling Water Systems 20 4.1.10 Cooling Tower Systems 21 4.2 End-of-Pipe Treatment Technologies 21 4.2.1 Preliminary Treatment 21 4.2.2 Biological Treatment 22 4.2.3 Effluent Polishing 23 4.2.3 Activated Carbon Treatment 24 4.2.5 Technologies Used at EPA/OAR Survey Refineries 24 4.2.6 Performance of End-of-Pipe Systems 26 4.2.7 Storm Water Management 29 5. -

Global Aromatics Supply - Today and Tomorrow M
New Technologies and Alternative Feedstocks in Petrochemistry and Refining DGMK Conference October 9-11, 2013, Dresden, Germany Global Aromatics Supply - Today and Tomorrow M. Bender BASF SE, Ludwigshafen, Germany Abstract Aromatics are the essential building blocks for some of the largest petrochemical products in today’s use. To the vast majority they are consumed to produce intermediates for polymer products and, hence, contribute to our modern lifestyle. Their growth rates are expected to be in line with GDP growth in future. This contrasts the significantly lower growth rates of the primary sources for aromatics – fuel processing and steam cracking of naphtha fractions. A supply gap can be expected to open up in future for which creative solutions will be required. Introduction The three basic aromatic compounds, benzene, toluene and xylenes are produced on large industrial scale with a total volume of approx. 95 million metric tons per year in 2012. While benzene and xylenes are consumed each by about 40 million metric tons per year, toluene is used as a chemical feedstock in somewhat smaller volumes. Fig. 1: Essential Building Blocks for Tomorrow´s Petrochemicals DGMK-Tagungsbericht 2013-2, ISBN 978-3-941721-32-6 59 New Technologies and Alternative Feedstocks in Petrochemistry and Refining Benzene is mainly consumed for manufacturing polystyrene (PS) via ethylbenzene. Xylenes are mostly produced for making polyethylene terephthalate (PET) via terephthalic acid. Toluene itself is used as a solvent and to a smaller extent in the strongly growing production of toluene diisocyanate (TDI), a building block for polyurethanes. Most of the toluene, however, is converted into benzene and xylenes by catalytic transalkylation, thereby adding to the volumes of the two major aromatics. -

Olefins Recovery CRYO-PLUS(PDF 4.0
02 REFINING & PETROCHEMICAL EXPERIENCE Linde Process Plants, Inc. (LPP) has constructed more than twenty (20) CRYO-PLUS™ units since 1984. Proprietary Technology Higher Recovery with Less Energy Refinery Configuration Designed to be used in low-pressure hydrogen-bearing Some of the principal crude oil conversion processes are off-gas applications, the patented CRYO-PLUS™ process fluid catalytic cracking and catalytic reforming. Both recovers approximately 98% of the propylene and heavier processes convert crude products (naphtha and gas oils) components with less energy required than traditional into high-octane unleaded gasoline blending components liquid recovery processes. (reformate and FCC gasoline). Cracking and reforming processes produce large quantities of both saturated and unsaturated gases. Higher Product Yields The resulting incremental recovery of the olefins such as propylene and butylene by the CRYO-PLUS™ process means Excess Fuel Gas in Refineries that more feedstock is available for alkylation and polym- The additional gas that is produced overloads refinery erization. The result is an overall increase in production of gas recovery processes. As a result, large quantities of high-octane, zero sulfur, gasoline. propylene and propane (C3’s), and butylenes and butanes (C4’s) are being lost to the fuel system. Many refineries produce more fuel gas than they use and flaring of the excess gas is all too frequently the result. Our Advanced Design for Ethylene Recovery The CRYO-PLUS C2=™ technology was specifically designed to recover ethylene and heavier hydrocarbons from low-pressure hydrogen-bearing refinery off-gas streams. Our patented design has eliminated many of the problems associated with technologies that predate the CRYO-PLUS C2=™ technology. -

Environmental, Health, and Safety Guidelines for Petroleum Refining
This document is NO LONGER IN USE by the World Bank Group. The new version of the World Bank Group Environmental, Health and Safety Guidelines are available at http://www.ifc.org/ehsguidelines Environmental, Health, and Safety Guidelines PETROLEUM REFINING RAFT FOR ECOND UBLIC ONSULTATION MARCH D S P C — 2016 WORLD BANK GROUP Environmental, Health, and Safety Guidelines for Petroleum Refining Introduction 1. The Environmental, Health, and Safety (EHS) Guidelines are technical reference documents with general and industry-specific examples of Good International Industry Practice (GIIP)1. When one or more members of the World Bank Group are involved in a project, these EHS Guidelines are applied as required by their respective policies and standards. These industry sector EHS guidelines are designed to be used together with the General EHS Guidelines document, which provides guidance to users on common EHS issues potentially applicable to all industry sectors. For complex projects, use of multiple industry sector guidelines may be necessary. A complete list of industry-sector guidelines can be found at: www.ifc.org/ehsguidelines 2. The EHS Guidelines contain the performance levels and measures that are generally considered to be achievable in new facilities by existing technology at reasonable costs. Application of the EHS Guidelines to existing facilities may involve the establishment of site-specific targets, with an appropriate timetable for achieving them. 3. The applicability of the EHS Guidelines should be tailored to the hazards and risks established for each project on the basis of the results of an environmental assessment in which site-specific variables, such as host country context, assimilative capacity of the environment, and other project factors, are taken into account. -

AFPM 2019 Refining Capacity Report
American Fuel & Petrochemical Manufacturers afpm.org AFPM United States Refining and Storage Capacity Report January 1, 2019 Published August 2019 Refining Capacity Report January 1, 2019 Locations of U.S. Refineries 2019 PADD V PADD IV PADD II PADD I PADD III Large: Over 75,000 bbl/cd Small: Under 75,000 bbl/cd AFPM: We Make Progress Refining Capacity Report January 1, 2019 AFPM United States Refining and Storage Capacity Report The enclosed statistics provide U.S. refining and storage The following table lists the total U.S. refining capacity for the capacity data as reported by the DOE Energy Information past five years: Administration in their 2019 Refinery Capacity Report. These data, along with other DOE statistics, are also available January 1 Total Capacity in electronically. (See the note at the bottom for details). This report Thousands of Barrels Per Calendar Day is also available on the AFPM website (www.afpm.org) located within the Data & Reports dropdown menu at the top of the 2015 2016 2017 2018 2019 webpage by clicking the Data & Statistics link. U.S. Capacity 17,967 18,317 18,617 18,598 18,802 On January 1, 2019, there were 135a operable refineries in the United States with total crude distillation capacity of about 18.80 million barrels per calendar day (bbl/cd) and 19.96 Percentage Change in U.S. Capacity from million barrels per stream day (bbl/sd). Of these, 132 refineries Previous Year were operating on January 1, 2019, with operating capacity listed at 18.69 million bbl/cd and 19.83 million bbl/sd.