(HDS) Unit for Petroleum Naphtha at 3500 Barrels Per Day
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BENZENE Disclaimer
United States Office of Air Quality EPA-454/R-98-011 Environmental Protection Planning And Standards June 1998 Agency Research Triangle Park, NC 27711 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF BENZENE Disclaimer This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Mention of trade names and commercial products does not constitute endorsement or recommendation of use. EPA-454/R-98-011 ii TABLE OF CONTENTS Section Page LIST OF TABLES.....................................................x LIST OF FIGURES.................................................. xvi EXECUTIVE SUMMARY.............................................xx 1.0 PURPOSE OF DOCUMENT .......................................... 1-1 2.0 OVERVIEW OF DOCUMENT CONTENTS.............................. 2-1 3.0 BACKGROUND INFORMATION ...................................... 3-1 3.1 NATURE OF POLLUTANT..................................... 3-1 3.2 OVERVIEW OF PRODUCTION AND USE ......................... 3-4 3.3 OVERVIEW OF EMISSIONS.................................... 3-8 4.0 EMISSIONS FROM BENZENE PRODUCTION ........................... 4-1 4.1 CATALYTIC REFORMING/SEPARATION PROCESS................ 4-7 4.1.1 Process Description for Catalytic Reforming/Separation........... 4-7 4.1.2 Benzene Emissions from Catalytic Reforming/Separation .......... 4-9 4.2 TOLUENE DEALKYLATION AND TOLUENE DISPROPORTIONATION PROCESS ............................ 4-11 4.2.1 Toluene Dealkylation -

Material Safety Data Sheet
MATERIAL NAME: Heavy Straight MSDS # EPL-13 Run Naphtha MATERIAL SAFETY DATA SHEET SECTION 1 X PRODUCT AND COMPANY IDENTIFICATION FOR EMERGENCY SOURCE INFORMATION CONTACT: Explorer Pipeline Company ¾ (918) 493 - 5100 6846 South Canton ¾ CHEMTREC: (800) 424-9300 (24 hour contact) P.O. Box 2650 ¾ CANUTEC: (613) 996-6666 Tulsa, Oklahoma 74101 ¾ SETIQ: 91-800-00214 TRADE NAMES/SYNONYMS: Penex CHEMICAL FAMILY: Mixture of C - 4 EPL Code: 10 Raffinate C6, Hydrocarbons This material safety data sheet represents the composite characteristics and properties of fungible petroleum hydrocarbons and other related substances transported by explorer pipeline company. The information presented was compiled from one or more product shipper sources and is intended to provide health and safety guidance for these fungible products. Individual shipper and manufacturer MSDSs are available at Explorer Pipeline Company’s, Tulsa, Oklahoma, offices. SECTION 2 ; HAZARDS IDENTIFICATION )))))))))))))))))EMERGENCY OVERVIEW))))))))))))))))) Flammable Liquid!! ¾ Clear liquid with petroleum odor; ¾ Harmful or fatal if swallowed, inhaled or absorbed through skin. ¾ May cause CNS depression. ¾ Can produce skin irritation upon prolonged or repeated contact. ¾ Keep away from heat, sparks and open flame; ¾ Wash thoroughly after handling; ¾ Contains petroleum distillates! If swallowed, do not induce vomiting since aspiration into the lungs will cause chemical pneumonia; ¾ Avoid breathing vapors or mist; ¾ Use only with adequate ventilation; and ¾ Obtain prompt medical attention. Keep Out of Reach of Children! )))))))))))))))))))))))))))))))))))))))))))))) SECTION 3 W COMPOSITION/INFORMATION OF INGREDIENTS INGREDIENT CAS NUMBER PERCENTAGE (%) Mixture of C4-C6 hydrocarbons 64741-70-4 100% ACUTE SUMMARY OF ACUTE HAZARDS: Harmful or fatal if swallowed, inhaled or absorbed through the skin. May cause CNS depression. -

Platinum Catalysts in Petroleum Refining
Platinum Catalysts in Petroleum Refining By S. W. Curry, B.s., M.B.A. Universal Oil Products Company, Des Plnines, Illinois Rejbriiiirig processes using platinunz catalysts have beconie oj- major importance in petroleum rejining during the past seven years. The?. enable the octane rating of naphthas to be greatly increased, am1 cue more economicnl than any other rejiningyrocess for the production of high octane gasoline. In this article the general nature of the processes is described and the Platforniing process is considwed iti iiiore rletnil. Platinum in any form was virtually unused years. The end is by no means in sight, in the petroleum industry until 1949. Then since the trend in octane number requirement, Universal Oil Products Company introduced particularly for automobiles, has continued it on an unprecedented scale as the active to creep upward year by year. catalytic agent in its Platforming process for To have advocated the use of 400 ounces catalytically upgrading low octane petroleum of a noble metal, selling at about $70 per naphthas to high quality products. ounce at that time, in a catalyst charge for a Prior to the installation of the first UOP single small commercial refinery unit, would Platforming unit, platinum was found chiefly doubtless have been branded prior to 1949 in laboratories in the oil industry. In sharp as the impractical idea of a dreamer. UOP's contrast with 1949, platinum today may be announcement surprised many in the oil regarded as a most essential item in the pro- industry for that matter. duction of high octane gasoline for automo- Even after the first Platformer had been biles and piston-engine aircraft. -

Sds – Safety Data Sheet
Effective Date: 08/29/16 Replaces Revision: 07/01/13, 06/29/09 NON-EMERGENCY TELEPHONE 24-HOUR CHEMTREC EMERGENCY TELEPHONE 610-866-4225 800-424-9300 SDS – SAFETY DATA SHEET 1. Identification Product Identifier: PETROLEUM ETHER Synonyms: Ligroin, VM&P Naphtha, Benzin, Petroleum Naphtha, Naphtha ASTM, Petroleum Spirits, Petroleum Ether of varying boiling point ranges from 20 to 75C (68 to 167F) Chemical Formula: Not applicable Recommended Use of the Chemical and Restrictions On Use: Laboratory Reagent Manufacturer / Supplier: Puritan Products; 2290 Avenue A, Bethlehem, PA 18017 Phone: 610-866-4225 Emergency Phone Number: 24-Hour Chemtrec Emergency Telephone 800-424-9300 2. Hazard(s) Identification Classification of the Substance or Mixture: Flammable liquids (Category 2) Germ cell mutagenicity (Category 1B) Carcinogenicity (Category 1A) Aspiration hazard (Category 1) Risk Phrases: R11: Highly flammable. R20: Harmful by inhalation. R22: Harmful if swallowed. R45: May cause cancer. R65: Harmful: may cause lung damage if swallowed. Label Elements: Trade Name: PETROLEUM ETHER Signal Word: Danger Hazard Statements: H225: Highly flammable liquid and vapor. H304: May be fatal if swallowed and enters airways. H340: May cause genetic defects. H350: May cause cancer. PETROLEUM ETHER Page 1 of 6 Precautionary Statements: P201: Obtain special instructions before use. P210: Keep away from heat / sparks / open flames / hot surfaces. No smoking. P301 + P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor / physician. P308 + P313: If exposed or concerned: Get medical advice / attention. P331: DO NOT induce vomiting. 3. Composition / Information on Ingredients CAS Number: 8032-32-4 EC Number: 232-453-7 Index Number: 649-263-00-9 Molecular Weight: 87-114 g/mol Chemical Ingredient CAS Number EC Number Percent Hazardous Characterization Naphtha, VM & P 8032-32-4 232-453-7 90 - 100% Yes Substance 4. -

Marathon Petroleum Naphtha Straight Run Light
SAFETY DATA SHEET SDS ID NO.: 0133MAR020 Revision Date 05/21/2015 1. IDENTIFICATION Product Name: Marathon Petroleum Naphtha, Straight Run Light Synonym: BTX Naphtha; Light Splitter Overhead Naphtha; Light Straight Run Naphtha; Naphtha Light Straight Run Product Code: 0133MAR020 Chemical Family: Aliphatic Naphtha Recommended Use: Feedstock. Restrictions on Use: All others. Manufacturer, Importer, or Responsible Party Name and Address: MARATHON PETROLEUM COMPANY LP 539 South Main Street Findlay, OH 45840 SDS information: 1-419-421-3070 Emergency Telephone: 1-877-627-5463 2. HAZARD IDENTIFICATION Classification OSHA Regulatory Status This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Flammable liquids Category 1 Skin corrosion/irritation Category 2 Germ cell mutagenicity Category 1B Carcinogenicity Category 1A Reproductive toxicity Category 2 Specific target organ toxicity (single exposure) Category 3 Specific target organ toxicity (repeated exposure) Category 1 Category 2 Aspiration toxicity Category 1 Acute aquatic toxicity Category 2 Chronic aquatic toxicity Category 2 Hazards Not Otherwise Classified (HNOC) Static accumulating flammable liquid Label elements EMERGENCY OVERVIEW SDS ID NO.: 0133MAR020 Product name: Marathon Petroleum Naphtha, Straight Run Light Page 1 of 19 0133MAR020 Marathon Petroleum Naphtha, Straight Revision Date 05/21/2015 Run Light _____________________________________________________________________________________________ Danger EXTREMELY FLAMMABLE LIQUID AND -

Optimize Refinery Hydrotreating & Catalytic Reforming Using Fast On
— ABB MEASUREMENT & ANALYTICS | APPlicatiON NOte Optimize refinery hydrotreating & catalytic reforming Using fast on-line simulated distillation GCs PGC5009 fast gas chromatograph The advantage of on-line fast simulated distillation. Measurement made easy — The challenge Catalytic reforming is a major conversion process in Industry | Refining Improve your control Improving profitability requires refiners to reduce petroleum refinery and petrochemical industries. strategies. the impact of market price volatility and product The process converts low octane naphthas into environmental compliance requirements by higher octane reformate products for gasoline adopting better control strategies. Better control blending and aromatic rich reformate for aromatic strategies enable refiners to handle a wide range of production. Hydrotreating is an important step to feedstocks while maintaining smooth operations remove unwanted sulfur from streams that are used and intermediate product qualities, both key to as feeds to some refinery units. Catalytic reforming sustainable margins and reliable operation. Most and isomerization are examples of refining traditional process and lab gas chromatographs as processes that require low sulfur feeds. The catalyst well as D86 distillation devices do not provide the in these processes are platinum based and sulfur required fast and reliable measurement feedback compounds are catalyst poisons. Controlling the due to long cycle times and poor repeatability. feed distillation properties enable control on not only the product properties but also the monitoring The ABB PGC5009 fast on-line gas chromatograph of the sulfur compounds. Intermediate streams used answers the challenge by providing superior process to feed hydrotreater do not have a uniform amount chromatography for simulate distillation in less than of sulfur compounds across their boiling point range 5 minutes. -

Rhea Van Gijzel
Eindhoven University of Technology MASTER Energy analysis and plant design for ethylene production from naphtha and natural gas van Gijzel, R.A. Award date: 2017 Link to publication Disclaimer This document contains a student thesis (bachelor's or master's), as authored by a student at Eindhoven University of Technology. Student theses are made available in the TU/e repository upon obtaining the required degree. The grade received is not published on the document as presented in the repository. The required complexity or quality of research of student theses may vary by program, and the required minimum study period may vary in duration. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain Process Engineering Multiphase Reactors group (SMR) Department of Chemical Engineering and Chemistry Den Dolech 2, 5612 AZ Eindhoven P.O. Box 513, 5600 MB Eindhoven The Netherlands www.tue.nl Graduation committee Prof. Dr. Ir. M. van Sint Annaland Dr. F. Gallucci Dr. V. Spallina (supervisor) Energy analysis and plant design for ethylene production Dr. T. Noël (external member) I. Campos Velarde from naphtha and natural gas Author R.A. van Gijzel MSc. -

Simulation and Modeling of Catalytic Reforming Process
Petroleum & Coal ISSN 1337-7027 Available online at www.vurup.sk/petroleum-coal Petroleum & Coal 54 (1) 76-84, 2012 SIMULATION AND MODELING OF CATALYTIC REFORMING PROCESS Aboalfazl Askari*, Hajir Karimi, M.Reza Rahimi, Mehdi Ghanbari Chemical engineering department, School of engineering,Yasouj University,Yasouj 75918-74831, Iran; [email protected] Received July 26, 2011, Accepted January 5, 2012 Abstract One of the most important processes in oil refineries is catalytic reforming unit in which high octane gasoline is produced. The catalytic reforming unit by using Hysys-refinery software was simulated. The results are validated by operating data, which is taken from the Esfahan oil refinery catalytic reforming unit. Usually, in oil refineries, flow instability in composition of feedstock can affect the product quality. The attention of this paper was focused on changes of the final product flow rate and product’s octane number with respect to the changes in the feedstock composition. Also, the effects of temperature and pressure on the mentioned parameters was evaluated. Furthermore, in this study, Smith kinetic model was evaluated. The accuracy of this model was compared with the actual data and Hysys-refinery’s results. The results showed that if the feed stream of catalytic reforming unit supplied with the Heavy Isomax Naphtha can be increased, more than 20% of the current value, the flow rate and octane number of the final product will be increased. Also, we found that the variations of temperature and pressure, under operating condition of the reactors of this unit, has no effect on octane number and final product flow rate. -

Reforming for Btx
PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Menlo Park, California Abstract Process Economics Program Report No. 129 REFORMING FOR BTX (June 1980) This report reviews the reforming of naphtha to produce benzene, toluene, and xylenes. The fundamentals of the reforming operations are discussed in detail. The economics were developed for reforming of two naphthas; a paraffinic and a naphthenic. Also, the effect of reforming severity on the economics is studied. LAC SF PEP'77 WS Fw Report No. 129 REFORMING FOR BTX by FRANK B. WEST Contributions by LESLIE A. CARMICHAEL STANFORD FIELD KOON LING RING WALTER SEDRIKS May 1980 A private report by the PROCESS ECONOMICS PROGRAM Menlo Park, California 94025 For detailed marketing data and information, the reader is referred to one of the SRI programs specializing-in marketing research. The CHEMICAL ECONOMICS UANDROOK Program covers most major chemicals and chemical products produced in the United States and the WORLD PETROCHEMICALS Program covers major hydrocarbons and their derivatives on a worldwide basis. In addition, the SRI DIRECTCRY OF CHEMICAL PRODUCERS services provide detailed lists of chemical producers by company, prod- uct, and plant for the United States and Western Europe. ii CONTENTS 1 INTRODUCTION . 1 2 SUMMARY . 3 3 INDUSTRY STATUS . : .................... 9 Production Capacity .................... 9 4 GENERAL PROCESS CONSIDERATIONS ............... 17 Introduction. ....................... 17 Chemistry ......................... 18 General ......................... 18 Feed Pretreating Reactions ................ 20 Reforming Reactions ................... 21 Isomerization and Dehydrogenation of Naphthenes ..... 22 Isomerization and Dehydrocyclization of Paraffins .... 23 Isomerization, Dealkylation, and Disproportionation of Aromatics ...................... 27 Isomerization ...................... 27 0 Dealkylation ....................... 27 Disproportionation and Transalkylation .......... 28 Hydrocracking of Paraffins and Naphthenes ........ 29 Coke Formation on the Catalyst ............. -

Steam Cracking: Chemical Engineering
Steam Cracking: Kinetics and Feed Characterisation João Pedro Vilhena de Freitas Moreira Thesis to obtain the Master of Science Degree in Chemical Engineering Supervisors: Professor Doctor Henrique Aníbal Santos de Matos Doctor Štepánˇ Špatenka Examination Committee Chairperson: Professor Doctor Carlos Manuel Faria de Barros Henriques Supervisor: Professor Doctor Henrique Aníbal Santos de Matos Member of the Committee: Specialist Engineer André Alexandre Bravo Ferreira Vilelas November 2015 ii The roots of education are bitter, but the fruit is sweet. – Aristotle All I am I owe to my mother. – George Washington iii iv Acknowledgments To begin with, my deepest thanks to Professor Carla Pinheiro, Professor Henrique Matos and Pro- fessor Costas Pantelides for allowing me to take this internship at Process Systems Enterprise Ltd., London, a seven-month truly worthy experience for both my professional and personal life which I will certainly never forget. I would also like to thank my PSE and IST supervisors, who help me to go through this final journey as a Chemical Engineering student. To Stˇ epˇ an´ and Sreekumar from PSE, thank you so much for your patience, for helping and encouraging me to always keep a positive attitude, even when harder problems arose. To Prof. Henrique who always showed availability to answer my questions and to meet in person whenever possible. Gostaria tambem´ de agradecer aos meus colegas de casa e de curso Andre,´ Frederico, Joana e Miguel, com quem partilhei casa. Foi uma experienciaˆ inesquec´ıvel que atravessamos´ juntos e cer- tamente que a vossa presenc¸a diaria´ apos´ cada dia de trabalho ajudou imenso a aliviar as saudades de casa. -

5.1 Petroleum Refining1
5.1 Petroleum Refining1 5.1.1 General Description The petroleum refining industry converts crude oil into more than 2500 refined products, including liquefied petroleum gas, gasoline, kerosene, aviation fuel, diesel fuel, fuel oils, lubricating oils, and feedstocks for the petrochemical industry. Petroleum refinery activities start with receipt of crude for storage at the refinery, include all petroleum handling and refining operations and terminate with storage preparatory to shipping the refined products from the refinery. The petroleum refining industry employs a wide variety of processes. A refinery's processing flow scheme is largely determined by the composition of the crude oil feedstock and the chosen slate of petroleum products. The example refinery flow scheme presented in Figure 5.1-1 shows the general processing arrangement used by refineries in the United States for major refinery processes. The arrangement of these processes will vary among refineries, and few, if any, employ all of these processes. Petroleum refining processes having direct emission sources are presented on the figure in bold-line boxes. Listed below are 5 categories of general refinery processes and associated operations: 1. Separation processes a. Atmospheric distillation b. Vacuum distillation c. Light ends recovery (gas processing) 2. Petroleum conversion processes a. Cracking (thermal and catalytic) b. Reforming c. Alkylation d. Polymerization e. Isomerization f. Coking g. Visbreaking 3.Petroleum treating processes a. Hydrodesulfurization b. Hydrotreating c. Chemical sweetening d. Acid gas removal e. Deasphalting 4.Feedstock and product handling a. Storage b. Blending c. Loading d. Unloading 5.Auxiliary facilities a. Boilers b. Waste water treatment c. Hydrogen production d. -
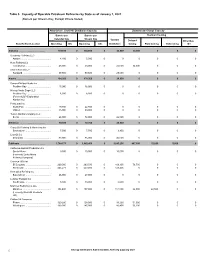
Table 3. Capacity of Operable Petroleum Refineries by State As of January 1, 2021 (Barrels Per Stream Day, Except Where Noted)
Table 3. Capacity of Operable Petroleum Refineries by State as of January 1, 2021 (Barrels per Stream Day, Except Where Noted) Atmospheric Crude Oil Distillation Capacity Downstream Charge Capacity Barrels per Barrels per Thermal Cracking Calendar Day Stream Day Vacuum Delayed Other/Gas State/Refiner/Location Operating Idle Operating Idle Distillation Coking Fluid Coking Visbreaking Oil Alabama......................................................... 139,600 0 145,600 0 54,000 34,000 0 0 0 Goodway Refining LLC ....................................................................................................................................................................................................Atmore 4,100 0 5,000 0 0 0 0 0 0 Hunt Refining Co ....................................................................................................................................................................................................Tuscaloosa 48,000 0 50,000 0 25,000 34,000 0 0 0 Shell Chemical LP ....................................................................................................................................................................................................Saraland 87,500 0 90,600 0 29,000 0 0 0 0 Alaska......................................................... 164,200 0 178,500 0 26,000 0 0 0 0 ConocoPhillips Alaska Inc ....................................................................................................................................................................................................Prudhoe