Simulation and Modeling of Catalytic Reforming Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(HDS) Unit for Petroleum Naphtha at 3500 Barrels Per Day
Available online at www.worldscientificnews.com WSN 9 (2015) 88-100 EISSN 2392-2192 Design Parameters for a Hydro desulfurization (HDS) Unit for Petroleum Naphtha at 3500 Barrels per Day Debajyoti Bose University of Petroleum & Energy Studies, College of Engineering Studies, P.O. Bidholi via- Prem Nagar, Dehradun 248007, India E-mail address: [email protected] ABSTRACT The present work reviews the setting up of a hydrodesulphurization unit for petroleum naphtha. Estimating all the properties of the given petroleum fraction including its density, viscosity and other parameters. The process flow sheet which gives the idea of necessary equipment to be installed, then performing all material and energy balance calculations along with chemical and mechanical design for the entire setup taking into account every instrument considered. The purpose of this review paper takes involves an industrial process, a catalytic chemical process widely used to remove sulfur (S) from naphtha. Keywords: hydro desulfurization, naphtha, petroleum, sulfur Relevance to Design Practice - The purpose of removing the sulfur is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion. World Scientific News 9 (2015) 88-100 1. INTRODUCTION Hydrodesulphurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur is to reduce the sulfur dioxide (SO2) emissions that result from various combustion practices. -

BENZENE Disclaimer
United States Office of Air Quality EPA-454/R-98-011 Environmental Protection Planning And Standards June 1998 Agency Research Triangle Park, NC 27711 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF BENZENE Disclaimer This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Mention of trade names and commercial products does not constitute endorsement or recommendation of use. EPA-454/R-98-011 ii TABLE OF CONTENTS Section Page LIST OF TABLES.....................................................x LIST OF FIGURES.................................................. xvi EXECUTIVE SUMMARY.............................................xx 1.0 PURPOSE OF DOCUMENT .......................................... 1-1 2.0 OVERVIEW OF DOCUMENT CONTENTS.............................. 2-1 3.0 BACKGROUND INFORMATION ...................................... 3-1 3.1 NATURE OF POLLUTANT..................................... 3-1 3.2 OVERVIEW OF PRODUCTION AND USE ......................... 3-4 3.3 OVERVIEW OF EMISSIONS.................................... 3-8 4.0 EMISSIONS FROM BENZENE PRODUCTION ........................... 4-1 4.1 CATALYTIC REFORMING/SEPARATION PROCESS................ 4-7 4.1.1 Process Description for Catalytic Reforming/Separation........... 4-7 4.1.2 Benzene Emissions from Catalytic Reforming/Separation .......... 4-9 4.2 TOLUENE DEALKYLATION AND TOLUENE DISPROPORTIONATION PROCESS ............................ 4-11 4.2.1 Toluene Dealkylation -

Converting Visbreakers to Delayed Cokers - an Opportunity for European Refiners
Converting Visbreakers to Delayed Cokers - An Opportunity for European Refiners European Coking.com Conference Sept. 30 - Oct. 2, 2008 Alex Broerse Lummus Technology a CB&I company © Lummus Technology Overview Introduction Delayed Coking Delayed Coking vs. Visbreaking Case Study Conclusions © Lummus Technology Converting Visbreakers to Delayed Cokers - 2 Fuel Oil Market General trend: reduction of sulfur content in fuel oil Typically 1.0-1.5 wt% S International Maritime Organization introduced SOx Emission Control Areas: . Sulfur content of fuel oil on board ships < 1.5 wt% . 1st SECA: Baltic Sea (effective 2006) . North Sea end of 2007 . More to follow Similar trend in other fuel oil application areas End of bunker fuel oil as sulfur sink? © Lummus Technology Converting Visbreakers to Delayed Cokers - 3 European Fuels Market Increased demand for ULS diesel Gradually decreasing fuel oil market Price gap between low sulfur crudes and opportunity crudes Re-evaluation of bottom-of-the-barrel strategy maximize diesel and minimize/eliminate fuel oil production What are the options? © Lummus Technology Converting Visbreakers to Delayed Cokers - 4 Bottom-of-the-Barrel Conversion Technologies Non Catalytic Catalytic Delayed coking Atm. / vac. resid hydrotreating Fluid / flexicoking Ebullated bed hydrocracking Gasification Resid FCC © Lummus Technology Converting Visbreakers to Delayed Cokers - 5 Lummus Capabilities for Bottom-of-the-Barrel Lummus Technology – Houston Delayed coking Resid FCC Chevron Lummus Global JV – Bloomfield Atmospheric/vacuum residue hydrotreating LC-FINING ebullated bed hydrocracking Lummus Technology – Bloomfield / The Hague Refinery planning studies (e.g., grassroots, revamps, processing of opportunity crudes) © Lummus TechnologyExtensive experience in heavy crude upgrade Converting Visbreakers to Delayed Cokers - 6 scenarios Overview Introduction Delayed Coking Delayed Coking vs. -

Visbreaking: a Technology of the Past and the Future
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Scientia Iranica C (2012) 19 (3), 569–573 Sharif University of Technology Scientia Iranica Transactions C: Chemistry and Chemical Engineering www.sciencedirect.com Visbreaking: A technology of the past and the future J.G. Speight ∗ CD&W Inc., 2476 Overland Road, Laramie, WY 82070, USA Received 18 August 2011; revised 1 December 2011; accepted 28 December 2011 KEYWORDS Abstract Because of their relative simplicity of design and straightforward thermal approach, visbreaking Petroleum refining; processes will not be ignored or absent from the refinery of the future. However, new and improved Visbreaking; approaches are important for the production of petroleum products. These will include advances in current Fouling. methods, minimization of process energy losses, and improved conversion efficiency. In addition, the use of additives to encourage the preliminary deposition of coke-forming constituents is also an option. Depending upon the additive, disposal of the process sediment can be achieved by a choice of methods. ' 2012 Sharif University of Technology. Production and hosting by Elsevier B.V. Open access under CC BY-NC-ND license. 1. Introduction variables are (1) feedstock type, (2) temperature, (3) pressure, and residence time, which need to be considered to control the extent of cracking. Balancing product yield and market demand, without the manufacture of large quantities of fractions having low com- 2. The visbreaking process mercial value, has long required processes for the conversion of hydrocarbons of one molecular weight range and/or struc- Visbreaking (viscosity reduction, viscosity breaking), a mild ture into some other molecular weight ranges and/or struc- form of thermal cracking, insofar as thermal reactions are tures. -

Optimize Refinery Hydrotreating & Catalytic Reforming Using Fast On
— ABB MEASUREMENT & ANALYTICS | APPlicatiON NOte Optimize refinery hydrotreating & catalytic reforming Using fast on-line simulated distillation GCs PGC5009 fast gas chromatograph The advantage of on-line fast simulated distillation. Measurement made easy — The challenge Catalytic reforming is a major conversion process in Industry | Refining Improve your control Improving profitability requires refiners to reduce petroleum refinery and petrochemical industries. strategies. the impact of market price volatility and product The process converts low octane naphthas into environmental compliance requirements by higher octane reformate products for gasoline adopting better control strategies. Better control blending and aromatic rich reformate for aromatic strategies enable refiners to handle a wide range of production. Hydrotreating is an important step to feedstocks while maintaining smooth operations remove unwanted sulfur from streams that are used and intermediate product qualities, both key to as feeds to some refinery units. Catalytic reforming sustainable margins and reliable operation. Most and isomerization are examples of refining traditional process and lab gas chromatographs as processes that require low sulfur feeds. The catalyst well as D86 distillation devices do not provide the in these processes are platinum based and sulfur required fast and reliable measurement feedback compounds are catalyst poisons. Controlling the due to long cycle times and poor repeatability. feed distillation properties enable control on not only the product properties but also the monitoring The ABB PGC5009 fast on-line gas chromatograph of the sulfur compounds. Intermediate streams used answers the challenge by providing superior process to feed hydrotreater do not have a uniform amount chromatography for simulate distillation in less than of sulfur compounds across their boiling point range 5 minutes. -

Environmental, Health, and Safety Guidelines for Petroleum Refining
ENVIRONMENTAL, HEALTH, AND SAFETY GUIDELINES PETROLEUM REFINING November 17, 2016 ENVIRONMENTAL, HEALTH, AND SAFETY GUIDELINES FOR PETROLEUM REFINING INTRODUCTION 1. The Environmental, Health, and Safety (EHS) Guidelines are technical reference documents with general and industry-specific examples of Good International Industry Practice (GIIP).1 When one or more members of the World Bank Group are involved in a project, these EHS Guidelines are applied as required by their respective policies and standards. These industry sector EHS Guidelines are designed to be used together with the General EHS Guidelines document, which provides guidance to users on common EHS issues potentially applicable to all industry sectors. For complex projects, use of multiple industry sector guidelines may be necessary. A complete list of industry sector guidelines can be found at: www.ifc.org/ehsguidelines. 2. The EHS Guidelines contain the performance levels and measures that are generally considered to be achievable in new facilities by existing technology at reasonable costs. Application of the EHS Guidelines to existing facilities may involve the establishment of site-specific targets, with an appropriate timetable for achieving them. 3. The applicability of the EHS Guidelines should be tailored to the hazards and risks established for each project on the basis of the results of an environmental assessment in which site-specific variables— such as host country context, assimilative capacity of the environment, and other project factors—are taken into account. The applicability of specific technical recommendations should be based on the professional opinion of qualified and experienced persons. 4. When host country regulations differ from the levels and measures presented in the EHS Guidelines, projects are expected to achieve whichever is more stringent. -

Reforming for Btx
PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Menlo Park, California Abstract Process Economics Program Report No. 129 REFORMING FOR BTX (June 1980) This report reviews the reforming of naphtha to produce benzene, toluene, and xylenes. The fundamentals of the reforming operations are discussed in detail. The economics were developed for reforming of two naphthas; a paraffinic and a naphthenic. Also, the effect of reforming severity on the economics is studied. LAC SF PEP'77 WS Fw Report No. 129 REFORMING FOR BTX by FRANK B. WEST Contributions by LESLIE A. CARMICHAEL STANFORD FIELD KOON LING RING WALTER SEDRIKS May 1980 A private report by the PROCESS ECONOMICS PROGRAM Menlo Park, California 94025 For detailed marketing data and information, the reader is referred to one of the SRI programs specializing-in marketing research. The CHEMICAL ECONOMICS UANDROOK Program covers most major chemicals and chemical products produced in the United States and the WORLD PETROCHEMICALS Program covers major hydrocarbons and their derivatives on a worldwide basis. In addition, the SRI DIRECTCRY OF CHEMICAL PRODUCERS services provide detailed lists of chemical producers by company, prod- uct, and plant for the United States and Western Europe. ii CONTENTS 1 INTRODUCTION . 1 2 SUMMARY . 3 3 INDUSTRY STATUS . : .................... 9 Production Capacity .................... 9 4 GENERAL PROCESS CONSIDERATIONS ............... 17 Introduction. ....................... 17 Chemistry ......................... 18 General ......................... 18 Feed Pretreating Reactions ................ 20 Reforming Reactions ................... 21 Isomerization and Dehydrogenation of Naphthenes ..... 22 Isomerization and Dehydrocyclization of Paraffins .... 23 Isomerization, Dealkylation, and Disproportionation of Aromatics ...................... 27 Isomerization ...................... 27 0 Dealkylation ....................... 27 Disproportionation and Transalkylation .......... 28 Hydrocracking of Paraffins and Naphthenes ........ 29 Coke Formation on the Catalyst ............. -

5.1 Petroleum Refining1
5.1 Petroleum Refining1 5.1.1 General Description The petroleum refining industry converts crude oil into more than 2500 refined products, including liquefied petroleum gas, gasoline, kerosene, aviation fuel, diesel fuel, fuel oils, lubricating oils, and feedstocks for the petrochemical industry. Petroleum refinery activities start with receipt of crude for storage at the refinery, include all petroleum handling and refining operations and terminate with storage preparatory to shipping the refined products from the refinery. The petroleum refining industry employs a wide variety of processes. A refinery's processing flow scheme is largely determined by the composition of the crude oil feedstock and the chosen slate of petroleum products. The example refinery flow scheme presented in Figure 5.1-1 shows the general processing arrangement used by refineries in the United States for major refinery processes. The arrangement of these processes will vary among refineries, and few, if any, employ all of these processes. Petroleum refining processes having direct emission sources are presented on the figure in bold-line boxes. Listed below are 5 categories of general refinery processes and associated operations: 1. Separation processes a. Atmospheric distillation b. Vacuum distillation c. Light ends recovery (gas processing) 2. Petroleum conversion processes a. Cracking (thermal and catalytic) b. Reforming c. Alkylation d. Polymerization e. Isomerization f. Coking g. Visbreaking 3.Petroleum treating processes a. Hydrodesulfurization b. Hydrotreating c. Chemical sweetening d. Acid gas removal e. Deasphalting 4.Feedstock and product handling a. Storage b. Blending c. Loading d. Unloading 5.Auxiliary facilities a. Boilers b. Waste water treatment c. Hydrogen production d. -

Modified Design for Vacuum Residue Processing
CT&F - Ciencia, Tecnología y Futuro - Vol. 4 Num. 2 Dec. 2010 MODIFIED DESIGN FOR VACUUM RESIDUE PROCESSING Sandro-Faruc González1*, Jesús Carrillo1, Manuel Núñez1, Luis-Javier Hoyos1* and Sonia-A. Giraldo2 1 Ecopetrol S. A. – Instituto Colombiano del Petróleo (ICP), A.A. 4185 Bucaramanga, Santander, Colombia 2 Universidad Industrial de Santander (UIS), Bucaramanga, Santander, Colombia e-mail: [email protected] [email protected] (Received, Feb. 16, 2010; Accepted, Nov. 30, 2010) ABSTRACT he world petroleum industry shows a decreasing in the oil reserves, specially the light kind. For this reason is very important to implement process schemes that give the possibility to improve the re- Tcuperation of valuable products of heavy oil. In this case the residue processing in each one of the petroleum refining stages earns great importance with the purpose of maximizing the quantity of fuel by barrel of feedstock processed. Therefore, it has been proposed the modification of the currently vacuum residues process scheme in the Ecopetrol´s Barrancabermeja refinery (DEMEX-Visbreaker-Hydroprocessing). That modi- fication consists in the incorporation of an additional Visbreaker stage, previous at DEMEX extraction stage. This investigation was developed with plant pilot tests combined with statistical models that predict the yield and the quality of the products obtained in the industrial plants. These models were developed by the Instituto Colombiano del Petróleo (ICP). The modified scheme Visbreaker I-DEMEX- Visbreaker II- Hydroprocessing, gives the possibility to increase the yield of middle distillates. Besides decrease the quantity of demetalized oil produced in DEMEX stage. This reduction is very favorable since environmental point of view, because it allows have a percentage of free capacity in the Hydroprocessing unit in order to removed sulfur of valuable products like Diesel and in this way to respect the environment law to this kind of fuel. -
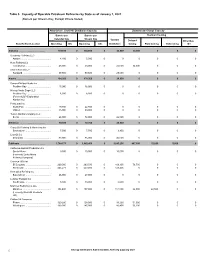
Table 3. Capacity of Operable Petroleum Refineries by State As of January 1, 2021 (Barrels Per Stream Day, Except Where Noted)
Table 3. Capacity of Operable Petroleum Refineries by State as of January 1, 2021 (Barrels per Stream Day, Except Where Noted) Atmospheric Crude Oil Distillation Capacity Downstream Charge Capacity Barrels per Barrels per Thermal Cracking Calendar Day Stream Day Vacuum Delayed Other/Gas State/Refiner/Location Operating Idle Operating Idle Distillation Coking Fluid Coking Visbreaking Oil Alabama......................................................... 139,600 0 145,600 0 54,000 34,000 0 0 0 Goodway Refining LLC ....................................................................................................................................................................................................Atmore 4,100 0 5,000 0 0 0 0 0 0 Hunt Refining Co ....................................................................................................................................................................................................Tuscaloosa 48,000 0 50,000 0 25,000 34,000 0 0 0 Shell Chemical LP ....................................................................................................................................................................................................Saraland 87,500 0 90,600 0 29,000 0 0 0 0 Alaska......................................................... 164,200 0 178,500 0 26,000 0 0 0 0 ConocoPhillips Alaska Inc ....................................................................................................................................................................................................Prudhoe -

Section 1 Introduction
SECTION 5.0 REFINING INDUSTRY DAMAGE MECHANISMS 5.1 General ................................................................................................................................ 1 5.1.1 Uniform or Localized Loss in Thickness Phenomena ................................................ 1 5.1.1.1 Amine Corrosion ......................................................................................................... 1 5.1.1.2 Ammonium Bisulfide Corrosion (Alkaline Sour Water) ......................................... 6 5.1.1.3 Ammonium Chloride Corrosion .............................................................................. 10 5.1.1.4 Hydrochloric Acid (HCl) Corrosion ......................................................................... 12 5.1.1.5 High Temp H2/H2S Corrosion ................................................................................... 15 5.1.1.6 Hydrofluoric (HF) Acid Corrosion ........................................................................... 19 5.1.1.7 Naphthenic Acid Corrosion (NAC) .......................................................................... 27 5.1.1.8 Phenol (Carbolic Acid) Corrosion ........................................................................... 31 5.1.1.9 Phosphoric Acid Corrosion ..................................................................................... 32 5.1.1.10 Sour Water Corrosion (Acidic) ............................................................................ 33 5.1.1.11 Sulfuric Acid Corrosion ...................................................................................... -

Preliminary Data Summary for the Petroleum Refining Category
Preliminary Data Summary for the Petroleum Refining Category United States Environmental Protection Agency Office of Water Engineering and Analysis Division 401 M Street, S.W. Washington, D.C. 20460 EPA 821-R-96-015 Apr il 1996 Table of Contents List of Figures iv List of Tables v Acknowledgments vii 1. Introduction 1 1.1 Background 1 1.2 Status of Categorical Regulations 1 1.3 Software Disk Available 4 2. Description of the Industry 5 2.1 Production Operations 5 2.1.1 Crude Oil and Product Storage 5 2.1.2 Crude Distillation 5 2.1.3 Cracking 6 2.1.4 Hydrocarbon Rebuilding 6 2.1.5 Hydrocarbon Rearrangements 6 2.1.6 Hydrotreating 6 2.1.7 Solvent Refining 7 2.1.8 Asphalt Production 7 2.1.9 Lubricating Oil Manufacture 7 2.1.10 Production of Petrochemicals 8 2.2 Industry Trends 8 3. Summary of Information Sources Used in This Study 13 3.1 Oil And Gas Journal Survey 13 3.2 EPA Office of Air and Radiation Questionnaire 13 3.3 Plant Visits 13 3.4 Permit Compliance System Data 13 3.5 Los Angeles County Sanitation Districts 14 3.6 Other Sewerage Authorities 14 3.7 Province of Ontario, Canada Petroleum Study 14 3.8 Other Data Sources 14 4. Treatment Technologies Used in The Industry 15 4.1 In-Plant Controls 16 4.1.1 Steam Stripping 16 4.1.2 Neutralization of Spent Acids and Caustics 18 4.1.3 Source Control 18 4.1.4 Wastewater Segregation 18 4.1.5 Boiler Condensate Recovery 19 4.1.6 Treated Effluent Reuse 19 i 4.1.7 Other General Measures 19 4.1.8 Cooling Water Systems 19 4.1.9 Once-Through Cooling Water Systems 20 4.1.10 Cooling Tower Systems 21 4.2 End-of-Pipe Treatment Technologies 21 4.2.1 Preliminary Treatment 21 4.2.2 Biological Treatment 22 4.2.3 Effluent Polishing 23 4.2.3 Activated Carbon Treatment 24 4.2.5 Technologies Used at EPA/OAR Survey Refineries 24 4.2.6 Performance of End-of-Pipe Systems 26 4.2.7 Storm Water Management 29 5.