Optimizing the Production of Benzene, Toluene and Xylene (Btx) from Nigerian Treated Heavy Naphthene Samples
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Refining/Petrochemical Integration-A New Paradigm Joseph C
Refining/Petrochemical Integration-A New Paradigm Joseph C. Gentry, Director - Global Licensing Engineered to Innovate® Refining/Petrochemical Integration-A New Paradigm Joseph C. Gentry, Director - Global Licensing, GTC Technology US, LLC Introduction The global trend in motor fuel consumption favors diesel over gasoline. There is a simultaneous increase in demand for various petrochemicals such as propylene and aromatics. Technology provid- ers have been successful to utilize the Fluid Catalytic Cracking (FCC) unit as a method to produce propylene by high severity operation, but the potential for other petrochemicals from these units has been neglected. Cat cracked gasoline contains a high level of olefins, some sulfur, and appreciable aromatics. Until now, the aromatics were not wanted due to the olefin and sulfur impurities in this stream. New technology is being commercialized to separate the aromatics from FCC gasoline in order to use them directly for downstream applications. Additionally, the olefin fraction can be converted into aromatics through a simple fixed-bed reaction system. Thus all of the gasoline components are efficiently made into high-value petrochemicals. This combination of technology is much more efficient than methods that some operators use, which recycles FCC gasoline to the reforming unit. Aromatics is a fast growing market. CMAI (acquired by IHS in 2011) in 2005 forecast benzene demand to grow at 4.1% per year between 2000 and 2020, resulting in total demand growth of 24.3 million tons.1 Mixed xylenes capacity will approximately double by 2020 to meet the strong antici- pated demand growth, mainly driven by the strong demand for polyester. -

BTX) / Hydrotreated Pygas (HPG)
Product Stewardship Summary Benzene, Toluene, Xylene Mixture (BTX) / Hydrotreated Pygas (HPG) The product stewardship summary is intended to give general information about the chemical or categories of chemicals addressed. It is not intended to provide an in-depth discussion of all health and safety information. Additional information on this chemical is available through the applicable Material Safety Data Sheet which must be consulted before using this chemical. The product stewardship summary does not supplant or replace required regulatory and/or legal communication documents. Chemical Identity: Benzene/Toluene/Xylene Mixture (BTX), also known as Hydrogenated Pyrolysis Gasoline (HPG), is a clear liquid with an aromatic odor. BTX/HPG is a co-product of ethylene production and is produced in closed systems that are monitored and controlled. HPG (BTX) is made by the double hydrogenation of raw pyrolysis gasoline (RPG), also known as debutanized aromatic concentrate (DAC). CAS Number: 68410-97-9 Synonyms:Pyrolysis Gasoline, High Benzene Naphtha, Aromatic Concentrate, Light Hydrotreated Distillate Product Uses: There are no consumer uses of BTX/HPG. BTX/HPG is used primarily as feedstock for benzene extraction. The remaining components are further separated into toluene and xylene. Benzene is primarily used for ethylbenzene to styrene and cumene to phenol production. The third largest use of benzene is in the production of cyclohexane, a nylon precursor. Toluene, the second largest aromatic in BTX/HPG, is used in refinery streams such as gasoline blending for its octane value. Xylenes may either be used in refinery streams for gasoline blending or further separated by isomers for chemical applications. Physical/Chemical Properties: BTX/HPG is classified by the U.S. -

(HDS) Unit for Petroleum Naphtha at 3500 Barrels Per Day
Available online at www.worldscientificnews.com WSN 9 (2015) 88-100 EISSN 2392-2192 Design Parameters for a Hydro desulfurization (HDS) Unit for Petroleum Naphtha at 3500 Barrels per Day Debajyoti Bose University of Petroleum & Energy Studies, College of Engineering Studies, P.O. Bidholi via- Prem Nagar, Dehradun 248007, India E-mail address: [email protected] ABSTRACT The present work reviews the setting up of a hydrodesulphurization unit for petroleum naphtha. Estimating all the properties of the given petroleum fraction including its density, viscosity and other parameters. The process flow sheet which gives the idea of necessary equipment to be installed, then performing all material and energy balance calculations along with chemical and mechanical design for the entire setup taking into account every instrument considered. The purpose of this review paper takes involves an industrial process, a catalytic chemical process widely used to remove sulfur (S) from naphtha. Keywords: hydro desulfurization, naphtha, petroleum, sulfur Relevance to Design Practice - The purpose of removing the sulfur is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion. World Scientific News 9 (2015) 88-100 1. INTRODUCTION Hydrodesulphurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur is to reduce the sulfur dioxide (SO2) emissions that result from various combustion practices. -

BENZENE Disclaimer
United States Office of Air Quality EPA-454/R-98-011 Environmental Protection Planning And Standards June 1998 Agency Research Triangle Park, NC 27711 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF BENZENE Disclaimer This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Mention of trade names and commercial products does not constitute endorsement or recommendation of use. EPA-454/R-98-011 ii TABLE OF CONTENTS Section Page LIST OF TABLES.....................................................x LIST OF FIGURES.................................................. xvi EXECUTIVE SUMMARY.............................................xx 1.0 PURPOSE OF DOCUMENT .......................................... 1-1 2.0 OVERVIEW OF DOCUMENT CONTENTS.............................. 2-1 3.0 BACKGROUND INFORMATION ...................................... 3-1 3.1 NATURE OF POLLUTANT..................................... 3-1 3.2 OVERVIEW OF PRODUCTION AND USE ......................... 3-4 3.3 OVERVIEW OF EMISSIONS.................................... 3-8 4.0 EMISSIONS FROM BENZENE PRODUCTION ........................... 4-1 4.1 CATALYTIC REFORMING/SEPARATION PROCESS................ 4-7 4.1.1 Process Description for Catalytic Reforming/Separation........... 4-7 4.1.2 Benzene Emissions from Catalytic Reforming/Separation .......... 4-9 4.2 TOLUENE DEALKYLATION AND TOLUENE DISPROPORTIONATION PROCESS ............................ 4-11 4.2.1 Toluene Dealkylation -

Optimize Refinery Hydrotreating & Catalytic Reforming Using Fast On
— ABB MEASUREMENT & ANALYTICS | APPlicatiON NOte Optimize refinery hydrotreating & catalytic reforming Using fast on-line simulated distillation GCs PGC5009 fast gas chromatograph The advantage of on-line fast simulated distillation. Measurement made easy — The challenge Catalytic reforming is a major conversion process in Industry | Refining Improve your control Improving profitability requires refiners to reduce petroleum refinery and petrochemical industries. strategies. the impact of market price volatility and product The process converts low octane naphthas into environmental compliance requirements by higher octane reformate products for gasoline adopting better control strategies. Better control blending and aromatic rich reformate for aromatic strategies enable refiners to handle a wide range of production. Hydrotreating is an important step to feedstocks while maintaining smooth operations remove unwanted sulfur from streams that are used and intermediate product qualities, both key to as feeds to some refinery units. Catalytic reforming sustainable margins and reliable operation. Most and isomerization are examples of refining traditional process and lab gas chromatographs as processes that require low sulfur feeds. The catalyst well as D86 distillation devices do not provide the in these processes are platinum based and sulfur required fast and reliable measurement feedback compounds are catalyst poisons. Controlling the due to long cycle times and poor repeatability. feed distillation properties enable control on not only the product properties but also the monitoring The ABB PGC5009 fast on-line gas chromatograph of the sulfur compounds. Intermediate streams used answers the challenge by providing superior process to feed hydrotreater do not have a uniform amount chromatography for simulate distillation in less than of sulfur compounds across their boiling point range 5 minutes. -

Simulation and Modeling of Catalytic Reforming Process
Petroleum & Coal ISSN 1337-7027 Available online at www.vurup.sk/petroleum-coal Petroleum & Coal 54 (1) 76-84, 2012 SIMULATION AND MODELING OF CATALYTIC REFORMING PROCESS Aboalfazl Askari*, Hajir Karimi, M.Reza Rahimi, Mehdi Ghanbari Chemical engineering department, School of engineering,Yasouj University,Yasouj 75918-74831, Iran; [email protected] Received July 26, 2011, Accepted January 5, 2012 Abstract One of the most important processes in oil refineries is catalytic reforming unit in which high octane gasoline is produced. The catalytic reforming unit by using Hysys-refinery software was simulated. The results are validated by operating data, which is taken from the Esfahan oil refinery catalytic reforming unit. Usually, in oil refineries, flow instability in composition of feedstock can affect the product quality. The attention of this paper was focused on changes of the final product flow rate and product’s octane number with respect to the changes in the feedstock composition. Also, the effects of temperature and pressure on the mentioned parameters was evaluated. Furthermore, in this study, Smith kinetic model was evaluated. The accuracy of this model was compared with the actual data and Hysys-refinery’s results. The results showed that if the feed stream of catalytic reforming unit supplied with the Heavy Isomax Naphtha can be increased, more than 20% of the current value, the flow rate and octane number of the final product will be increased. Also, we found that the variations of temperature and pressure, under operating condition of the reactors of this unit, has no effect on octane number and final product flow rate. -

Reforming for Btx
PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Menlo Park, California Abstract Process Economics Program Report No. 129 REFORMING FOR BTX (June 1980) This report reviews the reforming of naphtha to produce benzene, toluene, and xylenes. The fundamentals of the reforming operations are discussed in detail. The economics were developed for reforming of two naphthas; a paraffinic and a naphthenic. Also, the effect of reforming severity on the economics is studied. LAC SF PEP'77 WS Fw Report No. 129 REFORMING FOR BTX by FRANK B. WEST Contributions by LESLIE A. CARMICHAEL STANFORD FIELD KOON LING RING WALTER SEDRIKS May 1980 A private report by the PROCESS ECONOMICS PROGRAM Menlo Park, California 94025 For detailed marketing data and information, the reader is referred to one of the SRI programs specializing-in marketing research. The CHEMICAL ECONOMICS UANDROOK Program covers most major chemicals and chemical products produced in the United States and the WORLD PETROCHEMICALS Program covers major hydrocarbons and their derivatives on a worldwide basis. In addition, the SRI DIRECTCRY OF CHEMICAL PRODUCERS services provide detailed lists of chemical producers by company, prod- uct, and plant for the United States and Western Europe. ii CONTENTS 1 INTRODUCTION . 1 2 SUMMARY . 3 3 INDUSTRY STATUS . : .................... 9 Production Capacity .................... 9 4 GENERAL PROCESS CONSIDERATIONS ............... 17 Introduction. ....................... 17 Chemistry ......................... 18 General ......................... 18 Feed Pretreating Reactions ................ 20 Reforming Reactions ................... 21 Isomerization and Dehydrogenation of Naphthenes ..... 22 Isomerization and Dehydrocyclization of Paraffins .... 23 Isomerization, Dealkylation, and Disproportionation of Aromatics ...................... 27 Isomerization ...................... 27 0 Dealkylation ....................... 27 Disproportionation and Transalkylation .......... 28 Hydrocracking of Paraffins and Naphthenes ........ 29 Coke Formation on the Catalyst ............. -

Cracking (Chemistry)
Cracking (chemistry) In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of a large alkane into smaller, more useful alkenes. Simply put, hydrocarbon cracking is the process of breaking a long chain of hydrocarbons into short ones. This process requires high temperatures.[1] More loosely, outside the field of petroleum chemistry, the term "cracking" is used to describe any type of splitting of molecules under the influence of heat, catalysts and solvents, such as in processes of destructive distillation or pyrolysis. Fluid catalytic cracking produces a high yield of petrol and LPG, while hydrocracking is a major source of jet fuel, Diesel fuel, naphtha, and again yields LPG. Contents History and patents Cracking methodologies Thermal cracking Steam cracking Fluid Catalytic cracking Hydrocracking Fundamentals See also Refinery using the Shukhov cracking References process, Baku, Soviet Union, 1934. External links History and patents Among several variants of thermal cracking methods (variously known as the "Shukhov cracking process", "Burton cracking process", "Burton-Humphreys cracking process", and "Dubbs cracking process") Vladimir Shukhov, a Russian engineer, invented and patented the first in 1891 (Russian Empire, patent no. 12926, November 7, 1891).[2] One installation was used to a limited extent in Russia, but development was not followed up. In the first decade of the 20th century the American engineers William Merriam Burton and Robert E. -

5.1 Petroleum Refining1
5.1 Petroleum Refining1 5.1.1 General Description The petroleum refining industry converts crude oil into more than 2500 refined products, including liquefied petroleum gas, gasoline, kerosene, aviation fuel, diesel fuel, fuel oils, lubricating oils, and feedstocks for the petrochemical industry. Petroleum refinery activities start with receipt of crude for storage at the refinery, include all petroleum handling and refining operations and terminate with storage preparatory to shipping the refined products from the refinery. The petroleum refining industry employs a wide variety of processes. A refinery's processing flow scheme is largely determined by the composition of the crude oil feedstock and the chosen slate of petroleum products. The example refinery flow scheme presented in Figure 5.1-1 shows the general processing arrangement used by refineries in the United States for major refinery processes. The arrangement of these processes will vary among refineries, and few, if any, employ all of these processes. Petroleum refining processes having direct emission sources are presented on the figure in bold-line boxes. Listed below are 5 categories of general refinery processes and associated operations: 1. Separation processes a. Atmospheric distillation b. Vacuum distillation c. Light ends recovery (gas processing) 2. Petroleum conversion processes a. Cracking (thermal and catalytic) b. Reforming c. Alkylation d. Polymerization e. Isomerization f. Coking g. Visbreaking 3.Petroleum treating processes a. Hydrodesulfurization b. Hydrotreating c. Chemical sweetening d. Acid gas removal e. Deasphalting 4.Feedstock and product handling a. Storage b. Blending c. Loading d. Unloading 5.Auxiliary facilities a. Boilers b. Waste water treatment c. Hydrogen production d. -

PACT™ Light Alkanes Upgrade to Octane and Aromatics
PACT™ Light Alkanes upgrade to Octane and Aromatics Jens Michael Poulsen Anthony Baldridge 1 NGL production is rapidly increasing million barrels per day 70-75% of Natural Gasoline/C5+ supply historically used as gasoline blendstock Traditional C5+ Use Gasoline Petchem Exports Ethanol denaturing US natural gas plant liquids production, EIA Can traditional outlets absorb the increased C5+ Supply? 2 Excess C5+/Natural Gasoline/Gas Condensate C5+ oversupply forces exports and depress prices U.S. EIA estimates: 60% increase in C5+/natural gasoline supply within the US from 2018 to 2022 with supply continuing to outpace demand Largest Outlets: 1. Gasoline blending. Losing volume and value due to Low Octane (≈70 RON) High RVP (>12 PSIG) Sulfur (100-200 ppmw) 2. Steam cracking. Eroding market for Natural Gasoline due to low (negative) margin vs C2 Saturated markets require export of excess hydrocarbon gas liquids, at depressed prices Traditional outlets are unable to absorb the increased C5+ Supply! 3 A partnership of complementary competences World-class Refiner World-class Catalysts • 14,000 Employees • Research • 65 Countries • Development • No. 23 on Fortune 500 • Manufacturing 4 Joint Development Partnership Iterative Catalyst Design Goal Setting • Well-defined Partnership roles (P66 & HT) and existing strong relations Identify • Frequent iterative feedback loops Catalyst Design Opportunities on all organizational levels (HT) (P66 & HT) • 50 catalyst iterations tested thousands of hours in laboratory and pilot unit (≈1 barrel/day) Data Sharing Catalyst Testing (P66) (P66) Consistent feedback loop by both sides provides improved performance 5 Balancing for Optimal Results Aromatics Light Olefins Fuel Gas Controlling Factors Products • Feed Composition • Conditions • Recycle Streams • Process Design • Catalyst Choice Optimal point(s) depends on specific case (feedstock, product preferences) 6 Catalyst Development & Improvements Desired Product Selectivity Catalyst Deactivation wt% % Conversion Loss/hr. -
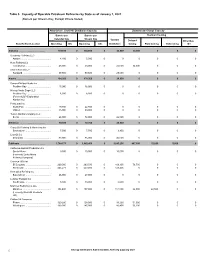
Table 3. Capacity of Operable Petroleum Refineries by State As of January 1, 2021 (Barrels Per Stream Day, Except Where Noted)
Table 3. Capacity of Operable Petroleum Refineries by State as of January 1, 2021 (Barrels per Stream Day, Except Where Noted) Atmospheric Crude Oil Distillation Capacity Downstream Charge Capacity Barrels per Barrels per Thermal Cracking Calendar Day Stream Day Vacuum Delayed Other/Gas State/Refiner/Location Operating Idle Operating Idle Distillation Coking Fluid Coking Visbreaking Oil Alabama......................................................... 139,600 0 145,600 0 54,000 34,000 0 0 0 Goodway Refining LLC ....................................................................................................................................................................................................Atmore 4,100 0 5,000 0 0 0 0 0 0 Hunt Refining Co ....................................................................................................................................................................................................Tuscaloosa 48,000 0 50,000 0 25,000 34,000 0 0 0 Shell Chemical LP ....................................................................................................................................................................................................Saraland 87,500 0 90,600 0 29,000 0 0 0 0 Alaska......................................................... 164,200 0 178,500 0 26,000 0 0 0 0 ConocoPhillips Alaska Inc ....................................................................................................................................................................................................Prudhoe -

Transformation of Bio-Oil Into BTX by Bio-Oil Catalytic Cracking
CHINESE JOURNAL OF CHEMICAL PHYSICS VOLUME 26, NUMBER 4 AUGUST 27, 2013 ARTICLE Transformation of Bio-oil into BTX by Bio-oil Catalytic Cracking Jiu-fang Zhu; Ji-cong Wang; Quan-xin Li¤ Department of Chemical Physics, University of Science and Technology of China, Hefei 230026, China (Dated: Received on April 11, 2013; Accepted on May 13, 2013) Production of benzene, toluene and xylenes (BTX) from bio-oil can provide basic feedstocks for the petrochemical industry. Catalytic conversion of bio-oil into BTX was performed by using di®erent pore characteristics zeolites (HZSM-5, HY-zeolite, and MCM-41). Based on the yield and selectivity of BTX, the production of aromatics decreases in the following order: HZSM-5>MCM-41>HY-zeolite. The highest BTX yield from bio-oil using HZSM-5 reached 33.1% with aromatics selectivity of 86.4%. The reaction conditions and catalyst characterization were investigated in detail to make clear the optimal operating parameters and the relation between the catalyst structure and the production of BTX. Key words: Bio-oil, BTX, Catalytic cracking I. INTRODUCTION as well as di®erent biomass feedstocks (husk, sawdust, sugarcane bagasse, cellulose, hemicellulose and lignin) Biomass conversion into benzene, toluene and xylenes with a lanthanum modi¯ed zeolite [11, 12]. Moreover, (BTX) can provide the basic feedstocks for the petro- the catalytic conversion of biomass or its derived feed- chemical industry, and these aromatics also serve as the stocks has been widely investigated with various zeolite most important aromatic platform molecules for the de- catalysts such as ZSM-5, HZSM-5, Y-zeolite, Beta zeo- velopment of high-end chemicals [1¡3].