Catalytic Reforming
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(HDS) Unit for Petroleum Naphtha at 3500 Barrels Per Day
Available online at www.worldscientificnews.com WSN 9 (2015) 88-100 EISSN 2392-2192 Design Parameters for a Hydro desulfurization (HDS) Unit for Petroleum Naphtha at 3500 Barrels per Day Debajyoti Bose University of Petroleum & Energy Studies, College of Engineering Studies, P.O. Bidholi via- Prem Nagar, Dehradun 248007, India E-mail address: [email protected] ABSTRACT The present work reviews the setting up of a hydrodesulphurization unit for petroleum naphtha. Estimating all the properties of the given petroleum fraction including its density, viscosity and other parameters. The process flow sheet which gives the idea of necessary equipment to be installed, then performing all material and energy balance calculations along with chemical and mechanical design for the entire setup taking into account every instrument considered. The purpose of this review paper takes involves an industrial process, a catalytic chemical process widely used to remove sulfur (S) from naphtha. Keywords: hydro desulfurization, naphtha, petroleum, sulfur Relevance to Design Practice - The purpose of removing the sulfur is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion. World Scientific News 9 (2015) 88-100 1. INTRODUCTION Hydrodesulphurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur is to reduce the sulfur dioxide (SO2) emissions that result from various combustion practices. -

BENZENE Disclaimer
United States Office of Air Quality EPA-454/R-98-011 Environmental Protection Planning And Standards June 1998 Agency Research Triangle Park, NC 27711 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF BENZENE Disclaimer This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Mention of trade names and commercial products does not constitute endorsement or recommendation of use. EPA-454/R-98-011 ii TABLE OF CONTENTS Section Page LIST OF TABLES.....................................................x LIST OF FIGURES.................................................. xvi EXECUTIVE SUMMARY.............................................xx 1.0 PURPOSE OF DOCUMENT .......................................... 1-1 2.0 OVERVIEW OF DOCUMENT CONTENTS.............................. 2-1 3.0 BACKGROUND INFORMATION ...................................... 3-1 3.1 NATURE OF POLLUTANT..................................... 3-1 3.2 OVERVIEW OF PRODUCTION AND USE ......................... 3-4 3.3 OVERVIEW OF EMISSIONS.................................... 3-8 4.0 EMISSIONS FROM BENZENE PRODUCTION ........................... 4-1 4.1 CATALYTIC REFORMING/SEPARATION PROCESS................ 4-7 4.1.1 Process Description for Catalytic Reforming/Separation........... 4-7 4.1.2 Benzene Emissions from Catalytic Reforming/Separation .......... 4-9 4.2 TOLUENE DEALKYLATION AND TOLUENE DISPROPORTIONATION PROCESS ............................ 4-11 4.2.1 Toluene Dealkylation -

National Academy of Sciences July 1, 1979 Officers
NATIONAL ACADEMY OF SCIENCES JULY 1, 1979 OFFICERS Term expires President-PHILIP HANDLER June 30, 1981 Vice-President-SAUNDERS MAC LANE June 30, 1981 Home Secretary-BRYCE CRAWFORD,JR. June 30, 1983 Foreign Secretary-THOMAS F. MALONE June 30, 1982 Treasurer-E. R. PIORE June 30, 1980 Executive Officer Comptroller Robert M. White David Williams COUNCIL Abelson, Philip H. (1981) Markert,C. L. (1980) Berg, Paul (1982) Nierenberg,William A. (1982) Berliner, Robert W. (1981) Piore, E. R. (1980) Bing, R. H. (1980) Ranney, H. M. (1980) Crawford,Bryce, Jr. (1983) Simon, Herbert A. (1981) Friedman, Herbert (1982) Solow, R. M. (1980) Handler, Philip (1981) Thomas, Lewis (1982) Mac Lane, Saunders (1981) Townes, Charles H. (1981) Malone, Thomas F. (1982) Downloaded by guest on September 30, 2021 SECTIONS The Academyis divided into the followingSections, to which membersare assigned at their own choice: (11) Mathematics (31) Engineering (12) Astronomy (32) Applied Biology (13) Physics (33) Applied Physical and (14) Chemistry Mathematical Sciences (15) Geology (41) Medical Genetics Hema- (16) Geophysics tology, and Oncology (21) Biochemistry (42) Medical Physiology, En- (22) Cellularand Develop- docrinology,and Me- mental Biology tabolism (23) Physiological and Phar- (43) Medical Microbiology macologicalSciences and Immunology (24) Neurobiology (51) Anthropology (25) Botany (52) Psychology (26) Genetics (53) Social and Political Sci- (27) Population Biology, Evo- ences lution, and Ecology (54) Economic Sciences In the alphabetical list of members,the numbersin parentheses, followingyear of election, indicate the respective Class and Section of the member. CLASSES The members of Sections are grouped in the following Classes: I. Physical and Mathematical Sciences (Sections 11, 12, 13, 14, 15, 16). -

E Helsinki Forum and East-West Scientific Exchange
[E HELSINKI FORUM AND EAST-WEST SCIENTIFIC EXCHANGE JOINT HEARING BEFORE THE SUBCOMMITTEE ON SCIENCE, RESEARCH AND TECHNOLOGY OF THE COMMITTEE ON SCIENCE AND TECHNOLOGY AND THE Sul COMMITTEE ON INTERNATIONAL SECURITY AND SCIENTIFIC AFFAIRS OF THE COMMITTEE ON FOREIGN AFFAIRS HOUSE OF REPRESENTATIVES AND THE COMMISSION ON SECURITY AND COOPERATION IN EUROPE NINETY-SIXTH CONGRESS SECOND SESSION JANUARY 31, 1980 [No. 89] (Committee on Science and Technology) ted for the use of the Committee on Science and Technology and the Committee on Foreign Affairs U.S. GOVERNMENT PRINTING OFFICE 421 0 WASHINGTON: 1980 COMMITTEE ON SCIENCE AND TECHNOLOGY DON FUQUA, Florida, Chairman ROBERT A. ROE, New Jersey JOHN W. WYDLER, New York MIKE McCORMACK, Washington LARRY WINN. JR., Kansas GEORGE E. BROWN, JR., California BARRY M. GOLDWATER, JR., California JAMES H. SCHEUER, New York HAMILTON FISH, JS., New York RICHARD L. OTTINGER, New York MANUEL LUJAN, JR., New Mexico TOM HARKIN, Iowa HAROLD C. HOLLENBECK, New Jersey JIM LLOYD, California ROBERT K. DORNAN, California JEROME A. AMBRO, New York ROBERT S. WALKER, Pennsylvania MARILYN LLOYD BOUQUARD, Tennessee EDWIN B. FORSYTHE, NeW Jersey JAMES J. BLANCHARD, Michigan KEN KRAMER, Colorado DOUG WALGREN, Pennsylvania WILLIAM CARNEY, New York RONNIE G. FLIPPO, Alabama ROBERT W. DAVIS, Michigan DAN GLICKMAN, Kansas TOBY ROTH, Wisconsin ALBERT GORE, JR., Tennessee DONALD LAWRENCE RITTER, WES WATKINS, Oklahoma Pennsylvania ROBERT A. YOUNG, Missouri BILL ROYER, California RICHARD C. WHITE, Texas HAROLD L. VOLKMER, Missouri DONALD J. PEASE, Ohio HOWARD WOLPE, Michigan NICHOLAS MAVROULES, Massachusetts BILL NELSON, Florida BERYL ANTHONY, JR., Arkansas STANLEY N. LUNDINE, New York ALLEN E. -

Optimize Refinery Hydrotreating & Catalytic Reforming Using Fast On
— ABB MEASUREMENT & ANALYTICS | APPlicatiON NOte Optimize refinery hydrotreating & catalytic reforming Using fast on-line simulated distillation GCs PGC5009 fast gas chromatograph The advantage of on-line fast simulated distillation. Measurement made easy — The challenge Catalytic reforming is a major conversion process in Industry | Refining Improve your control Improving profitability requires refiners to reduce petroleum refinery and petrochemical industries. strategies. the impact of market price volatility and product The process converts low octane naphthas into environmental compliance requirements by higher octane reformate products for gasoline adopting better control strategies. Better control blending and aromatic rich reformate for aromatic strategies enable refiners to handle a wide range of production. Hydrotreating is an important step to feedstocks while maintaining smooth operations remove unwanted sulfur from streams that are used and intermediate product qualities, both key to as feeds to some refinery units. Catalytic reforming sustainable margins and reliable operation. Most and isomerization are examples of refining traditional process and lab gas chromatographs as processes that require low sulfur feeds. The catalyst well as D86 distillation devices do not provide the in these processes are platinum based and sulfur required fast and reliable measurement feedback compounds are catalyst poisons. Controlling the due to long cycle times and poor repeatability. feed distillation properties enable control on not only the product properties but also the monitoring The ABB PGC5009 fast on-line gas chromatograph of the sulfur compounds. Intermediate streams used answers the challenge by providing superior process to feed hydrotreater do not have a uniform amount chromatography for simulate distillation in less than of sulfur compounds across their boiling point range 5 minutes. -

UNITED STATES PATENT OFFICE 2,478,916 REFORMING Process Vladimir Haensel, Clarendon Hills, and Curtis F
Patented Aug. 16, 1949 2.478,916 UNITED STATES PATENT OFFICE 2,478,916 REFORMING PRocess Vladimir Haensel, Clarendon Hills, and Curtis F. Gerald, Riverside, E., assignors to Universa Oil Products Company, Chicago, I., a corporation of Delaware No Drawing. Application December 21, 1946, Serial No. 71,659 13 Claims. (Cl 196-50) 2 This invention relates to a reforming process mally liquid hydrocarbons substantially or con and more particularly to a process for the re pletely into normally gaseous hydrocarbons. forming of a saturated gasoline fraction in the The desired selective cracking generally com presence of a particular catalyst and under se prises the removal of methyl, ethyl and, to a lected conditions of operation. lesser extent, propyl groups, in the form of The saturated gasoline fraction to be treat methane, ethane and propane as will be herein ed in accordance with the present invention after described. However, the removal of these comprise straight run gasolines, natural gaso radicals is controlled so that not more than. One lines, etc. The gasoline fraction may be a full or possibly two of such radicals are removed boiling range gasoline having an initial boiling O from a given molecule. For example, heptane point within the range of about 50 to about may be reduced to hexane, nonane to Octane or 90 F. and an end boiling point within the heptane, etc. On the other hand uncontrolled range of about 375 to about 4.25° F., or it may or non-selective cracking will result in the de be a selected fraction thereof which usually will composition of normally liquid hydrocarbons be a higher boiling fraction, commonly referred into normally gaseous hydrocarbons as, for ex to as naphtha, and generally having an initial ample, by the continued demethylation of nor boiling point of from about 150 to about 250 F. -

Catalysis: Change for the Better
Catalysis: Change for the Better Grade Level petroleum into the high-level fuels that we rely on for nearly all our transportation Elementary through middle school with adult needs. It also produces "aromatic" assistance. petrochemicals, the raw materials used in the manufacture of plastics and fibers. High school This new idea in catalysts, and the ideas Materials that followed, have made our fuel more efficient, environmentally-friendly and easier Ordinary sugar cubes and cheaper to produce. It has also made Matches the plastics industry more environmentally- Ash friendly. Metal plate or can lid In 1998, Vladimir Haensel received the Charles Stark Draper Prize, a $450,000 Discussion award, for his work. This award is the "Nobel Prize" of engineering. it is presented by the Sometimes a chemical reaction won't take National Academy of Engineering, with an place, or will proceed very slowly, unless endowment from the Charles Stark Draper some other substance is added. That other Laboratory. Charles Stark Draper was an substance is called a catalyst, which engineer and "father" of modern inertial hastens the action but is not part of it and guidance systems, used in commercial does not change. Catalysts are widely used aircraft, space vehicles, strategic missiles, in the chemical industry to speed up and submarines. Draper also developed the reactions. An important use of catalysts is in sophisticated navigational system that the making of gasoline from crude oil. landed the Apollo astronauts on the moon and returned them safely to earth. In 1949 at Universal Oil Products (now UOP), chemical engineer Vladimir Haensel developed a revolutionary process called Platforming. -

Simulation and Modeling of Catalytic Reforming Process
Petroleum & Coal ISSN 1337-7027 Available online at www.vurup.sk/petroleum-coal Petroleum & Coal 54 (1) 76-84, 2012 SIMULATION AND MODELING OF CATALYTIC REFORMING PROCESS Aboalfazl Askari*, Hajir Karimi, M.Reza Rahimi, Mehdi Ghanbari Chemical engineering department, School of engineering,Yasouj University,Yasouj 75918-74831, Iran; [email protected] Received July 26, 2011, Accepted January 5, 2012 Abstract One of the most important processes in oil refineries is catalytic reforming unit in which high octane gasoline is produced. The catalytic reforming unit by using Hysys-refinery software was simulated. The results are validated by operating data, which is taken from the Esfahan oil refinery catalytic reforming unit. Usually, in oil refineries, flow instability in composition of feedstock can affect the product quality. The attention of this paper was focused on changes of the final product flow rate and product’s octane number with respect to the changes in the feedstock composition. Also, the effects of temperature and pressure on the mentioned parameters was evaluated. Furthermore, in this study, Smith kinetic model was evaluated. The accuracy of this model was compared with the actual data and Hysys-refinery’s results. The results showed that if the feed stream of catalytic reforming unit supplied with the Heavy Isomax Naphtha can be increased, more than 20% of the current value, the flow rate and octane number of the final product will be increased. Also, we found that the variations of temperature and pressure, under operating condition of the reactors of this unit, has no effect on octane number and final product flow rate. -

Reforming for Btx
PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Menlo Park, California Abstract Process Economics Program Report No. 129 REFORMING FOR BTX (June 1980) This report reviews the reforming of naphtha to produce benzene, toluene, and xylenes. The fundamentals of the reforming operations are discussed in detail. The economics were developed for reforming of two naphthas; a paraffinic and a naphthenic. Also, the effect of reforming severity on the economics is studied. LAC SF PEP'77 WS Fw Report No. 129 REFORMING FOR BTX by FRANK B. WEST Contributions by LESLIE A. CARMICHAEL STANFORD FIELD KOON LING RING WALTER SEDRIKS May 1980 A private report by the PROCESS ECONOMICS PROGRAM Menlo Park, California 94025 For detailed marketing data and information, the reader is referred to one of the SRI programs specializing-in marketing research. The CHEMICAL ECONOMICS UANDROOK Program covers most major chemicals and chemical products produced in the United States and the WORLD PETROCHEMICALS Program covers major hydrocarbons and their derivatives on a worldwide basis. In addition, the SRI DIRECTCRY OF CHEMICAL PRODUCERS services provide detailed lists of chemical producers by company, prod- uct, and plant for the United States and Western Europe. ii CONTENTS 1 INTRODUCTION . 1 2 SUMMARY . 3 3 INDUSTRY STATUS . : .................... 9 Production Capacity .................... 9 4 GENERAL PROCESS CONSIDERATIONS ............... 17 Introduction. ....................... 17 Chemistry ......................... 18 General ......................... 18 Feed Pretreating Reactions ................ 20 Reforming Reactions ................... 21 Isomerization and Dehydrogenation of Naphthenes ..... 22 Isomerization and Dehydrocyclization of Paraffins .... 23 Isomerization, Dealkylation, and Disproportionation of Aromatics ...................... 27 Isomerization ...................... 27 0 Dealkylation ....................... 27 Disproportionation and Transalkylation .......... 28 Hydrocracking of Paraffins and Naphthenes ........ 29 Coke Formation on the Catalyst ............. -

5.1 Petroleum Refining1
5.1 Petroleum Refining1 5.1.1 General Description The petroleum refining industry converts crude oil into more than 2500 refined products, including liquefied petroleum gas, gasoline, kerosene, aviation fuel, diesel fuel, fuel oils, lubricating oils, and feedstocks for the petrochemical industry. Petroleum refinery activities start with receipt of crude for storage at the refinery, include all petroleum handling and refining operations and terminate with storage preparatory to shipping the refined products from the refinery. The petroleum refining industry employs a wide variety of processes. A refinery's processing flow scheme is largely determined by the composition of the crude oil feedstock and the chosen slate of petroleum products. The example refinery flow scheme presented in Figure 5.1-1 shows the general processing arrangement used by refineries in the United States for major refinery processes. The arrangement of these processes will vary among refineries, and few, if any, employ all of these processes. Petroleum refining processes having direct emission sources are presented on the figure in bold-line boxes. Listed below are 5 categories of general refinery processes and associated operations: 1. Separation processes a. Atmospheric distillation b. Vacuum distillation c. Light ends recovery (gas processing) 2. Petroleum conversion processes a. Cracking (thermal and catalytic) b. Reforming c. Alkylation d. Polymerization e. Isomerization f. Coking g. Visbreaking 3.Petroleum treating processes a. Hydrodesulfurization b. Hydrotreating c. Chemical sweetening d. Acid gas removal e. Deasphalting 4.Feedstock and product handling a. Storage b. Blending c. Loading d. Unloading 5.Auxiliary facilities a. Boilers b. Waste water treatment c. Hydrogen production d. -
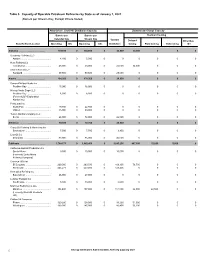
Table 3. Capacity of Operable Petroleum Refineries by State As of January 1, 2021 (Barrels Per Stream Day, Except Where Noted)
Table 3. Capacity of Operable Petroleum Refineries by State as of January 1, 2021 (Barrels per Stream Day, Except Where Noted) Atmospheric Crude Oil Distillation Capacity Downstream Charge Capacity Barrels per Barrels per Thermal Cracking Calendar Day Stream Day Vacuum Delayed Other/Gas State/Refiner/Location Operating Idle Operating Idle Distillation Coking Fluid Coking Visbreaking Oil Alabama......................................................... 139,600 0 145,600 0 54,000 34,000 0 0 0 Goodway Refining LLC ....................................................................................................................................................................................................Atmore 4,100 0 5,000 0 0 0 0 0 0 Hunt Refining Co ....................................................................................................................................................................................................Tuscaloosa 48,000 0 50,000 0 25,000 34,000 0 0 0 Shell Chemical LP ....................................................................................................................................................................................................Saraland 87,500 0 90,600 0 29,000 0 0 0 0 Alaska......................................................... 164,200 0 178,500 0 26,000 0 0 0 0 ConocoPhillips Alaska Inc ....................................................................................................................................................................................................Prudhoe -

Preliminary Data Summary for the Petroleum Refining Category
Preliminary Data Summary for the Petroleum Refining Category United States Environmental Protection Agency Office of Water Engineering and Analysis Division 401 M Street, S.W. Washington, D.C. 20460 EPA 821-R-96-015 Apr il 1996 Table of Contents List of Figures iv List of Tables v Acknowledgments vii 1. Introduction 1 1.1 Background 1 1.2 Status of Categorical Regulations 1 1.3 Software Disk Available 4 2. Description of the Industry 5 2.1 Production Operations 5 2.1.1 Crude Oil and Product Storage 5 2.1.2 Crude Distillation 5 2.1.3 Cracking 6 2.1.4 Hydrocarbon Rebuilding 6 2.1.5 Hydrocarbon Rearrangements 6 2.1.6 Hydrotreating 6 2.1.7 Solvent Refining 7 2.1.8 Asphalt Production 7 2.1.9 Lubricating Oil Manufacture 7 2.1.10 Production of Petrochemicals 8 2.2 Industry Trends 8 3. Summary of Information Sources Used in This Study 13 3.1 Oil And Gas Journal Survey 13 3.2 EPA Office of Air and Radiation Questionnaire 13 3.3 Plant Visits 13 3.4 Permit Compliance System Data 13 3.5 Los Angeles County Sanitation Districts 14 3.6 Other Sewerage Authorities 14 3.7 Province of Ontario, Canada Petroleum Study 14 3.8 Other Data Sources 14 4. Treatment Technologies Used in The Industry 15 4.1 In-Plant Controls 16 4.1.1 Steam Stripping 16 4.1.2 Neutralization of Spent Acids and Caustics 18 4.1.3 Source Control 18 4.1.4 Wastewater Segregation 18 4.1.5 Boiler Condensate Recovery 19 4.1.6 Treated Effluent Reuse 19 i 4.1.7 Other General Measures 19 4.1.8 Cooling Water Systems 19 4.1.9 Once-Through Cooling Water Systems 20 4.1.10 Cooling Tower Systems 21 4.2 End-of-Pipe Treatment Technologies 21 4.2.1 Preliminary Treatment 21 4.2.2 Biological Treatment 22 4.2.3 Effluent Polishing 23 4.2.3 Activated Carbon Treatment 24 4.2.5 Technologies Used at EPA/OAR Survey Refineries 24 4.2.6 Performance of End-of-Pipe Systems 26 4.2.7 Storm Water Management 29 5.