Organic Reactions Summary Alkenes, Alkynes and Variations for Use As A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prebiological Evolution and the Metabolic Origins of Life
Prebiological Evolution and the Andrew J. Pratt* Metabolic Origins of Life University of Canterbury Keywords Abiogenesis, origin of life, metabolism, hydrothermal, iron Abstract The chemoton model of cells posits three subsystems: metabolism, compartmentalization, and information. A specific model for the prebiological evolution of a reproducing system with rudimentary versions of these three interdependent subsystems is presented. This is based on the initial emergence and reproduction of autocatalytic networks in hydrothermal microcompartments containing iron sulfide. The driving force for life was catalysis of the dissipation of the intrinsic redox gradient of the planet. The codependence of life on iron and phosphate provides chemical constraints on the ordering of prebiological evolution. The initial protometabolism was based on positive feedback loops associated with in situ carbon fixation in which the initial protometabolites modified the catalytic capacity and mobility of metal-based catalysts, especially iron-sulfur centers. A number of selection mechanisms, including catalytic efficiency and specificity, hydrolytic stability, and selective solubilization, are proposed as key determinants for autocatalytic reproduction exploited in protometabolic evolution. This evolutionary process led from autocatalytic networks within preexisting compartments to discrete, reproducing, mobile vesicular protocells with the capacity to use soluble sugar phosphates and hence the opportunity to develop nucleic acids. Fidelity of information transfer in the reproduction of these increasingly complex autocatalytic networks is a key selection pressure in prebiological evolution that eventually leads to the selection of nucleic acids as a digital information subsystem and hence the emergence of fully functional chemotons capable of Darwinian evolution. 1 Introduction: Chemoton Subsystems and Evolutionary Pathways Living cells are autocatalytic entities that harness redox energy via the selective catalysis of biochemical transformations. -

1.1 10 Oxidation of Alcohols and Aldehydes
1.1 10 Oxidation of alcohols and aldehydes By the end of this spread, you should be able to … 1Describe the oxidation of primary alcohols to form aldehydes and carboxylic acids. 1Describe the oxidation of secondary alcohols to form ketones. 1Describe the oxidation of aldehydes to form carboxylic acids. Key definition Oxidation of alcohols You will recall from your AS chemistry studies that primary and secondary alcohols can A redox reaction is one in which both reduction and oxidation take place. be oxidised using an oxidising agent. s !SUITABLEOXIDISINGAGENTISASOLUTIONCONTAININGACIDIlEDDICHROMATEIONS + 2− H /Cr2O7 . s 4HEOXIDISINGMIXTURECANBEMADEFROMPOTASSIUMDICHROMATE +2Cr2O7, and sulfuric acid, H2SO. During the reaction, the acidified potassium dichromate changes from orange to green. Remember that tertiary alcohols are not oxidised by acidified dichromate ions. Primary alcohols Primary alcohols can be oxidised to aldehydes and to carboxylic acids (see AS Chemistry spread 2.2.3). H H H O H O Oxidation Oxidation H CCOH H CC H CC H H H H H OH Primary alcohol Aldehyde Carboxylic acid Figure 1 Ethanol oxidised to ethanal, and finally to ethanoic acid The equation for the oxidation of ethanol to the aldehyde ethanal is shown below. Note that the oxidising agent is shown as [O] – this simplifies the equation. CH3CH2OH + [O] }m CH3CHO + H2O When preparing aldehydes in the laboratory, you will need to distil the aldehyde from the reaction mixture as it is formed. This prevents the aldehyde from being oxidised further to a carboxylic acid. Key definition When making the carboxylic acid, the reaction mixture is usually heated under reflux before distilling the product off. -
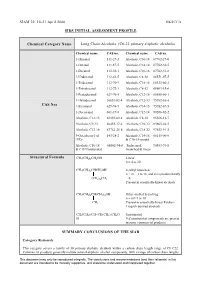
Long Chain Alcohols (C6-22 Primary Aliphatic Alcohols)
SIAM 22, 18-21 April 2006 UK/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Long Chain Alcohols (C6-22 primary aliphatic alcohols) Chemical name CAS no. Chemical name CAS no. 1-Hexanol 111-27-3 Alcohols, C16-18 67762-27-0 1-Octanol 111-87-5 Alcohols, C14-18 67762-30-5 1-Decanol 112-30-1 Alcohols, C10-16 67762-41-8 1-Undecanol 112-42-5 Alcohols, C8-18 68551-07-5 1-Tridecanol 112-70-9 Alcohols, C14-16 68333-80-2 1-Tetradecanol 112-72-1 Alcohols, C6-12 68603-15-6 1-Pentadecanol 629-76-5 Alcohols, C12-16 68855-56-1 1-Hexadecanol 36653-82-4 Alcohols, C12-13 75782-86-4 CAS Nos 1-Eicosanol 629-96-9 Alcohols, C14-15 75782-87-5 1-Docosanol 661-19-8 Alcohols, C12-14 80206-82-2 Alcohols, C12-15 63393-82-8 Alcohols, C8-10 85566-12-7 Alcohols, C9-11 66455-17-2 Alcohols, C10-12 85665-26-5 Alcohols, C12-18 67762-25-8 Alcohols, C18-22 97552-91-5 9-Octadecen-1-ol 143-28-2 Alcohols, C14-18. 68155-00-0 (9Z)- & C16-18-unsatd Alcohols, C16-18 68002-94-8 Tridecanol, 90583-91-8 & C18 Unsaturated branched & linear Structural Formula CH3(CH2)nCH2OH Linear n = 4 to 20 CH3(CH2)nCHCH2OH 2-Alkyl branched n + m = 3 to 18, and m is predominantly (CH2)mCH3 = 0. Present in essentially-linear alcohols CH3(CH2)nCH(CH2)mOH Other-methyl branching n + m= 9 or 10 CH3 Present in essentially-linear Fischer- Tropsch derived alcohols CH3(CH2)7CH=CH(CH2)7CH2O Unsaturated H 9-Z unsaturated components are present in some commercial products. -

Organic Chemistry PEIMS Code: N1120027 Abbreviation: ORGCHEM Number of Credits That May Be Earned: 1/2-1
Course: Organic Chemistry PEIMS Code: N1120027 Abbreviation: ORGCHEM Number of credits that may be earned: 1/2-1 Brief description of the course (150 words or less): Organic chemistry is an introductory course that is designed for the student who intends to continue future study in the sciences. The student will learn the concepts and applications of organic chemistry. Topics covered include aliphatic and aromatic compounds, alcohols, aldehydes, ketones, acids, ethers, amines, spectra, and stereochemistry. A brief introduction into biochemistry is also provided. The laboratory experiments will familiarize the student with the important laboratory techniques, specifically spectroscopy. Traditional high school chemistry courses focus on the inorganic aspects of chemistry whereas organic chemistry introduces the student to organic compounds and their properties, mechanisms of formations, and introduces the student to laboratory techniques beyond the traditional high school chemistry curriculum. Essential Knowledge and Skills of the course: (a) General requirements. This course is recommended for students in Grades 11-12. The recommended prerequisite for this course is AP Chemistry. (b) Introduction. Organic chemistry is designed to introduce students to the fundamental concepts of organic chemistry and key experimental evidence and data, which support these concepts. Students will learn to apply these data and concepts to chemical problem solving. Additionally, students will learn that organic chemistry is still evolving by reading about current breakthroughs in the field. Finally, students will gain appreciation for role that organic chemistry plays in modern technological developments in diverse fields, ranging from biology to materials science. (c) Knowledge and skills. (1) The student will be able to write both common an IUPAC names for the hydrocarbon. -

HISTORICAL ASPECTS of ORGANIC CHEMISTRY TEACHING (On the Example of Chemistry Direction of Higher Educational Institutions)
Journal of Critical Reviews ISSN- 2394-5125 Vol 7, Issue 13, 2020 CONCEPTUAL - HISTORICAL ASPECTS OF ORGANIC CHEMISTRY TEACHING (on the example of chemistry direction of Higher educational institutions) Rajabov Khudayor Madrimovich Uzbekistan, Khorasm region, Rajabov Khudayor Madrimovich, Doctor of philosophy (PhD) on pedagogical sciences, Urganch State University E-mail: [email protected] Received: 16.04.2020 Revised: 18.05.2020 Accepted: 13.06.2020 Abstract. The article reveals the historical approach on the teaching of organic chemistry in pedagogical universities. The development of modern trends and general competencies of students on the basis of historical approach, the creation of educational motivation, the focus on independent research and training of creative specialists are the most important directions. In this regard, it is important to improve the technology for organizing the educational process on the basis of historical principles in the system of training future chemistry teachers, to improve the mechanisms on the methodological support, to fulfill the system of competencies and cyclic diagnostics. Keywords: historical approach, organic chemistry, conceptual - historical aspects, professional competence, principle of historicism, methodology, self-improvement, innovation, non-historical, positive attitude, extracurricular activities. © 2020 by Advance Scientific Research. This is an open-access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/) DOI: http://dx.doi.org/10.31838/jcr.07.13.129 -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

The Arene–Alkene Photocycloaddition
The arene–alkene photocycloaddition Ursula Streit and Christian G. Bochet* Review Open Access Address: Beilstein J. Org. Chem. 2011, 7, 525–542. Department of Chemistry, University of Fribourg, Chemin du Musée 9, doi:10.3762/bjoc.7.61 CH-1700 Fribourg, Switzerland Received: 07 January 2011 Email: Accepted: 23 March 2011 Ursula Streit - [email protected]; Christian G. Bochet* - Published: 28 April 2011 [email protected] This article is part of the Thematic Series "Photocycloadditions and * Corresponding author photorearrangements". Keywords: Guest Editor: A. G. Griesbeck benzene derivatives; cycloadditions; Diels–Alder; photochemistry © 2011 Streit and Bochet; licensee Beilstein-Institut. License and terms: see end of document. Abstract In the presence of an alkene, three different modes of photocycloaddition with benzene derivatives can occur; the [2 + 2] or ortho, the [3 + 2] or meta, and the [4 + 2] or para photocycloaddition. This short review aims to demonstrate the synthetic power of these photocycloadditions. Introduction Photocycloadditions occur in a variety of modes [1]. The best In the presence of an alkene, three different modes of photo- known representatives are undoubtedly the [2 + 2] photocyclo- cycloaddition with benzene derivatives can occur, viz. the addition, forming either cyclobutanes or four-membered hetero- [2 + 2] or ortho, the [3 + 2] or meta, and the [4 + 2] or para cycles (as in the Paternò–Büchi reaction), whilst excited-state photocycloaddition (Scheme 2). The descriptors ortho, meta [4 + 4] cycloadditions can also occur to afford cyclooctadiene and para only indicate the connectivity to the aromatic ring, and compounds. On the other hand, the well-known thermal [4 + 2] do not have any implication with regard to the reaction mecha- cycloaddition (Diels–Alder reaction) is only very rarely nism. -

C12-14 Amine Oxide
Date: 06/06/2017 Environmental Fact Sheet (#20) C12‐14 Amine Oxide (Lauryldimethylamine oxide – C12‐14 AO) oleochemical non‐ionic surfactant Substance Identification Amines, C12‐14 (even IUPAC Name numbered)‐alkyldimethyl, N‐ CAS Number 308062‐28‐4 oxides Other Names Lauryldimethylamine oxide UVCB substance (substances Structural formula: of Unknown or Variable composition, Complex reaction products or Molecular Formula Biological materials), no univocal molecular formula available Physical/Chemical Properties [1] Molecular Weight 229.4‐257.5 g/mol Physical state Solid Appearance White powder Density 716 kg/m3 at 23 °C Melting Points 126‐136 °C Boiling point Study scientifically unjustified Flash Point Study technically not feasible Vapour Pressure 1.7*10‐06 and 7.5*10‐05 Pa at 25°C Water Solubility 409.5 g/L Flammability Non flammable Explosive Properties Study scientifically unjustified Surface Tension 34.1 mN/m at 20 °C Octanol/water Partition coefficient log KOW =1.85‐2.69 (Kow) Lauryldimethylamine oxide in a neutral aqueous solution is regarded as a non‐ionic surfactant. Amine oxides are produced by the reaction of tertiary amines such as Alkyldimethylamine with hydrogen peroxide in a two‐phase system containing a large volume of water yielding dilute products typically containing <35% active [5]. The reaction is a continuous process. In the primary step the Product and Process tertiary amine, a hydrogen peroxide solution, deionized water as well as the catalyst and its chelate Description are pumped continuously at controlled rates into a stirred reactor. This oxidation reaction is exothermic and yields approximately 95% of the desired product. To generate more product, the outflow of the first reactor is passed to a second reactor, which extends the yield of the amine oxide to approximately 99%. -

Aromatic Compounds
OCR Chemistry A Aromatic Compounds Aromatic Compounds Naming Aromatic compounds contain one or more benzene rings (while aliphatic compounds do not contain benzene rings). Another term for a compound containing a benzene ring is arene. The basic benzene ring, C6H6 is commonly represented as a hexagon with a ring inside. You should be aware that there is a hydrogen at each corner although this is not normally shown. N.B. it is OK to use benzene in this form in structural formulae too! Arenes occur naturally in many substances, and are present in coal and crude oil. Aspirin, for example, is an aromatic compound, an arene: HO O O CH 3 O Naming of substances based on benzene follows familiar rules: CH Br NO 3 2 benzene methylbenzene bromobenzene nitrobenzene Numbers are needed to identify the positions of substituents. The carbons around the ring are numbered from 1-6 consecutively and the name which gives the lowest number(s) is chosen: CH3 CH3 Cl Br Cl Br 2-bromomethylbenzene 4-bromomethylbenzene 1,2-dichlorobenzene Cl Cl Cl 1,3,5-trichlorobenzene Page 1 OCR Chemistry A Aromatic Compounds Determining the structure of benzene (historical) 1825 – substance first isolated by Michael Faraday, who also determined its empirical formula as CH 1834 – RFM of 78 and molecular formula of C6H6 determined H H H C C C Much speculation over the structure. Many suggested structures like: C C C H H H 1865 – Kekulé publishes suggestion of a ring with alternating double and single bonds: H H C H C C displayed C C skeletal H C H H This model persisted until 1922, but not all chemists accepted the structure because it failed to explain the chemical and physical properties of benzene fully: if C=C bonds were present as Kekulé proposed, then benzene would react like alkenes. -

Alkenes and Alkynes
02/21/2019 CHAPTER FOUR Alkenes and Alkynes H N O I Cl C O C O Cl F3C C Cl C Cl Efavirenz Haloprogin (antiviral, AIDS therapeutic) (antifungal, antiseptic) Chapter 4 Table of Content * Unsaturated Hydrocarbons * Introduction and hybridization * Alkenes and Alkynes * Benzene and Phenyl groups * Structure of Alkenes, cis‐trans Isomerism * Nomenclature of Alkenes and Alkynes * Configuration cis/trans, and cis/trans Isomerism * Configuration E/Z * Physical Properties of Hydrocarbons * Acid‐Base Reactions of Hydrocarbons * pka and Hybridizations 1 02/21/2019 Unsaturated Hydrocarbons • Unsaturated Hydrocarbon: A hydrocarbon that contains one or more carbon‐carbon double or triple bonds or benzene‐like rings. – Alkene: contains a carbon‐carbon double bond and has the general formula CnH2n. – Alkyne: contains a carbon‐carbon triple bond and has the general formula CnH2n‐2. Introduction Alkenes ● Hydrocarbons containing C=C ● Old name: olefins • Steroids • Hormones • Biochemical regulators 2 02/21/2019 • Alkynes – Hydrocarbons containing C≡C – Common name: acetylenes Unsaturated Hydrocarbons • Arene: benzene and its derivatives (Ch 9) 3 02/21/2019 Benzene and Phenyl Groups • We do not study benzene and its derivatives until Chapter 9. – However, we show structural formulas of compounds containing a phenyl group before that time. – The phenyl group is not reactive under any of the conditions we describe in chapters 5‐8. Structure of Alkenes • The two carbon atoms of a double bond and the four atoms bonded to them lie in a plane, with bond angles of approximately 120°. 4 02/21/2019 Structure of Alkenes • Figure 4.1 According to the orbital overlap model, a double bond consists of one bond formed by overlap of sp2 hybrid orbitals and one bond formed by overlap of parallel 2p orbitals. -

Low Ph Hair Conditioner Compositions Containing Amine Oxides
Europaisches Patentamt 3 European Patent Office @ Publication number: 0168 719 A? Office europeen des brevets r^Cm ® EUROPEAN PATENT APPLICATION @ Application number: 85108277.6 © Int. CI.4: A 61 K 7/075 @ Date of filing : 04.07.85 ® Priority: 20.07.84 US 632745 @ Applicant: Revlon, Inc., 767 Fifth Avenue, New York, N.Y.10022(US) @ Inventor: Gersteln, Terry, 12 Boston Post Road, East @ Date of publication of application: 22.01.86 Brunswick New Jersey (US) Bulletin 86/4 @ Representative : Schmidt-Evers, Jiirgen, Dipl.-lng. et al, Patentanwalte Dipl.-lng. H. Mitscherlich Dlpl.-lng. K. Gunschmann Dipl.-lng. Dr.rer.nat. W. Korber Dipl.-lng. J. Schmidt-Evers Dipl.-lng. W. Melzer Steinsdorf strasse 1 0, @ Designated Contracting States : CH DE FR GB IT LI D-8000 MUnchen 22 (DE) @ Low pH hair conditioner compositions containing amine oxides. The present invention relates to a cationic hair condition- ing composition comprising: (a) An amine oxide of the formula: wherein R1 and R2 are the same or different moieties and are selected from fewer (C1-C4) alkyl, alkoxy and hydroxy alkyl groups, and R3 is an alkyl group containing 8 to 22 carbon at- oms; and (b) water sufficient acid to provide pH for said composi- tion of from about 4.5 to about 2.4 or less and an amine oxide concentration from about 0.5% to about 10% based on the to- tal weight of said hair conditioning composition. This invention relates to hair conditioning compositions. More particularly, this invention relates to aqueous hair conditioning compositions that comprise relatively high molecular weight amine oxides at low pH's. -

Introduction to Alkenes and Alkynes in an Alkane, All Covalent Bonds
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene, however, only three σ bonds are formed from the alkene carbon -the carbon thus adopts an sp2 hybridization Ethene (common name ethylene) has a molecular formula of CH2CH2 Each carbon is sp2 hybridized with a σ bond to two hydrogens and the other carbon Hybridized orbital allows stronger bonds due to more overlap H H C C H H Structure of Ethylene In addition to the σ framework of ethylene, each carbon has an atomic p orbital not used in hybridization The two p orbitals (each with one electron) overlap to form a π bond (p bonds are not symmetric about the internuclear axis) π bonds are not as strong as σ bonds (in ethylene, the σ bond is ~90 Kcal/mol and the π bond is ~66 Kcal/mol) Thus while σ bonds are stable and very few reactions occur with C-C bonds, π bonds are much more reactive and many reactions occur with C=C π bonds Nomenclature of Alkenes August Wilhelm Hofmann’s attempt for systematic hydrocarbon nomenclature (1866) Attempted to use a systematic name by naming all possible structures with 4 carbons Quartane a alkane C4H10 Quartyl C4H9 Quartene e alkene C4H8 Quartenyl C4H7 Quartine i alkine → alkyne C4H6 Quartinyl C4H5 Quartone o C4H4 Quartonyl C4H3 Quartune u C4H2 Quartunyl C4H1 Wanted to use Quart from the Latin for 4 – this method was not embraced and BUT has remained Used English order of vowels, however, to name the groups